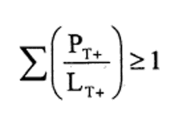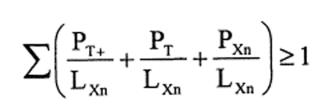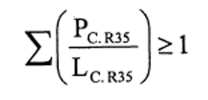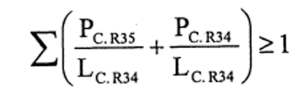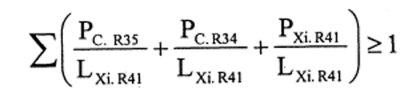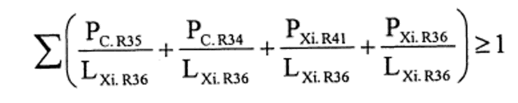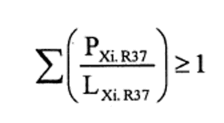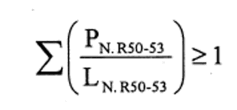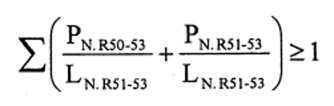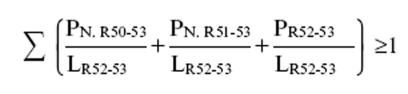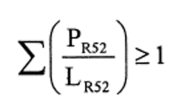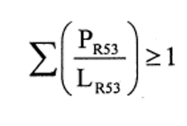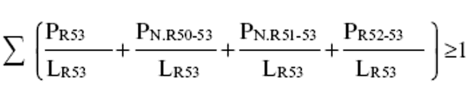- Y Diweddaraf sydd Ar Gael (Diwygiedig)
- Gwreiddiol (a wnaed Fel)
The Chemicals (Hazard Information and Packaging for Supply) Regulations 2009
You are here:
- Offerynnau Statudol y Deyrnas Unedig
- 2009 No. 716
- Whole Instrument
- Blaenorol
- Nesaf
Rhagor o Adnoddau
Status:
Dyma’r fersiwn wreiddiol (fel y’i gwnaed yn wreiddiol).
Statutory Instruments
2009 No. 716
HEALTH AND SAFETY
The Chemicals (Hazard Information and Packaging for Supply) Regulations 2009
Made
16th March 2009
Laid before Parliament
16th March 2009
Coming into force
6th April 2009
The Secretary of State is a Minister designated for the purpose of section 2(2) of the European Communities Act 1972(1) (“the 1972 Act”) in relation to the regulation and control of classification, packaging and labelling of dangerous substances and preparations(2), and for measures relating to consumer protection(3).
The Secretary of State makes these Regulations:
(a)in exercise of the powers conferred upon him by section 2(2) of the 1972 Act and by sections 15(1), (2), (3)(c), (4)(b), (6)(b), (8) and (9) and 82(3)(a) of, and paragraphs 1(1)(b) and (c), (4) and (5), 2(1), 3(2), 15(1) and 16 of Schedule 3 to, the Health and Safety at Work etc. Act 1974(4) (“the 1974 Act”); and
(b)for the purpose of giving effect without modifications to proposals submitted to him by the Health and Safety Executive under section 11(3)(5) of the 1974 Act after the carrying out by the said Executive of consultations in accordance with section 50(3) of that Act.
These Regulations make provision for a purpose mentioned in section 2(2) of the 1972 Act and it appears to the Secretary of State that it is expedient for references in these Regulations to—
the Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008(6) on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC(7) and 1999/45/EC(8), and amending Regulation (EC) No 1907/2006(9), be construed as including references to articles 6(5), 11(3), 12, 14, 18(3)(b), 23, 25 to 29, 35(2) second and third sub-paragraph and Annexes I to VII of that Regulation as amended from time to time; and
the Regulation (EC) No 689/2008 of the European Parliament and of the Council of 17 June 2008 concerning the export and import of dangerous chemicals(10) be construed as including references to Annexes I and V of that Regulation as those Annexes are amended from time to time.
PART 1INTRODUCTION
Citation, commencement and extent
1.—(1) These Regulations may be cited as the Chemicals (Hazard Information and Packaging for Supply) Regulations 2009 and shall come into force on 6th April 2009.
(2) These Regulations shall not extend to Northern Ireland.
Interpretation
2.—(1) In these Regulations—
“the 1974 Act” means the Health and Safety at Work etc. Act 1974;
“aerosol dispenser” means an article which consists of a non-reusable receptacle containing a gas compressed, liquefied or dissolved under pressure, with or without liquid, paste or powder and fitted with a release device allowing the contents to be ejected as solid or liquid particles in suspension in a gas, as a foam, paste or powder or in a liquid state;
“the approved classification and labelling guide” means the guide entitled “Approved Guide to the Classification and Labelling of Dangerous Substances and Dangerous Preparations (Fifth Edition)” approved by the Health and Safety Commission on 16th April 2002(11), as revised or re-issued from time to time;
“category of danger” means, in relation to a dangerous substance or dangerous preparation, one of the categories of danger specified in column 1 of Schedule 1;
“the CLP Regulation” means Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006, of which articles 6(5), 11(3), 12, 14, 18(3)(b), 23, 25 to 29, 35(2) second and third sub-paragraph and Annexes I to VII are as amended from time to time;
“Community workplace exposure limit” means, in respect of a substance, an exposure limit for that substance established in a Community instrument;
“dangerous preparation” means a preparation which is in one or more of the categories of danger specified in column 1 of Schedule 1;
“dangerous substance” means a substance—
which is listed in Table 3.2 of part 3 of Annex VI of the CLP Regulation; or
which, if it is not so listed, is in one or more of the categories of danger specified in column 1 of Schedule 1;
“EC number” means—
in the case of a dangerous substance that appears in Table 3.2 of part 3 of Annex VI of the CLP Regulation, the EC number specified in that list;
in the case of a dangerous substance that is not included in Table 3.2 of part 3 of Annex VI of the CLP Regulation or for which an EC number is not given in that list, the number for that substance specified in EINECS; or
in the case of a dangerous substance that is not a phase-in substance within the meaning of REACH, the number for that substance if it is listed in ELINCS;
“EEA Agreement” means the Agreement on the European Economic Area signed at Oporto on 2nd May 1992, as adjusted by the Protocol signed at Brussels on 17th March 1993 and adopted as respects the United Kingdom by the European Economic Area Act 1993(12);
“EEA State” means a state which is a contracting party to the EEA Agreement;
“EINECS” means the European Inventory of Existing Commercial Chemical Substances(13);
“ELINCS” means the European List of Notified Chemical Substances(14);
“enforcing authority” shall be construed in accordance with regulation 14;
“the Executive” means the Health and Safety Executive;
“indication of danger” means, in relation to a dangerous substance or dangerous preparation, one or more of the indications of danger referred to in column 1 of Schedule 2 and—
in the case of a dangerous substance listed in Table 3.2 of part 3 of Annex VI of the CLP Regulation, it is one or more of the indications of danger specified for that substance by a symbol-letter in that list;
in the case of any other dangerous substance or a dangerous preparation, it is one or more indications of danger determined in accordance with the classification of that substance or preparation in accordance with regulation 4 and the approved classification and labelling guide;
“plant protection product” has the same meaning as it has in regulation 2(1) of the Plant Protection Products Regulations 2005(15) and regulation 2(1) of the Plant Protection Products (Scotland) Regulations 2005(16);
“the Plant Protection Products Regulations” means the Plant Protection Products Regulations 2005 and the Plant Protection Products (Scotland) Regulations 2005;
“preparation” means a mixture or solution composed of two or more substances;
“radioactive substance” means a substance which contains one or more radionuclides whose activity or concentration cannot be disregarded as far as radiation protection is concerned;
“receptacle” means a container together with any material, wrapping and component, including any closure or fastener, associated with the container which enables the container to perform its containment function;
“REACH” means Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals;
“risk phrase” means a risk phrase listed in Annex III of Council Directive 67/548/EEC;
“safety phrase” means a safety phrase listed in Annex IV of Council Directive 67/548/EEC;
“substance” means a chemical element and its compounds in the natural state or obtained by any production process, including any additive necessary to preserve the stability of the product and any impurity deriving from the process used, but excluding any solvent which may be separated without affecting the stability of the substance or changing its composition;
“supply” in relation to a substance or preparation means making that substance or preparation available to another person and includes importation of the substance or preparation into Great Britain, and
“supplier” shall be construed accordingly.
(2) In these Regulations,
“package” means—
(a)subject to paragraph (3), the package in which a dangerous substance, dangerous preparation or preparation specified in regulation 11(3) is supplied, including the receptacle containing the dangerous substance or preparation in question; or
(b)a pallet or other device which enables more than one receptacle to be handled as a unit,
but does not include a container used to transport the dangerous substance or preparation unless that container is retained by the person to whom the dangerous substance or preparation is supplied for the purpose of storing that dangerous substance or preparation, and related expressions shall be construed accordingly.
(3) In the case of supply by way of retail sale, a package does not include any paper or plastic bag or other form of outer wrapping in which the package is placed when it is presented to the purchaser.
(4) In these Regulations—
(a)where reference is made to a quantity of a dangerous substance or dangerous preparation expressed in litres, that reference shall mean—
(i)in the case of a liquid, the volume in litres of that liquid;
(ii)in the case of a compressed gas, the volume in litres of the receptacle containing that gas; and
(iii)in the case of a compressed gas dissolved in a solvent, liquefied gas or solid, the same number of kilograms of that solvent, gas or solid; and
(b)for the purposes of aggregation, one kilogram of a solid shall be deemed to be equivalent to one litre of liquid or gas.
(5) In these Regulations—
(a)a risk phrase may be designated by the letter
“R” followed by a distinguishing number or combination of numbers; and
(b)a safety phrase may be designated by the letter “S” followed by a distinguishing number or combination of numbers.
Application
3.—(1) Subject to paragraphs (2) to (6), these Regulations shall apply to any dangerous substance or dangerous preparation.
(2) These Regulations shall not apply to a substance or preparation which is—
(a)intended for use as a medicinal product within the meaning of section 130 of the Medicines Act 1968(17);
(b)intended for use as a veterinary medical product within the meaning of regulation 2(1) of the Veterinary Medicines Regulations 2008(18);
(c)intended for use as an investigational medical product within the meaning of the Medicines for Human Use (Clinical Trials) Regulations 2004(19);
(d)specified in an order made under section 104 or 105 of the Medicines Act 1968 which is for the time being in force and which directs that specified provisions of that Act shall have effect in relation to medicinal products within the meaning of that Act;
(e)a controlled drug within the meaning of the Misuse of Drugs Act 1971(20) except that these Regulations shall apply to drugs which are excepted from section 4(1)(b) of that Act (which makes it unlawful to supply a controlled drug) by Regulations made under section 7(1)(a) of that Act;
(f)a cosmetic product within the meaning of the Cosmetic Products (Safety) Regulations 2008(21);
(g)in the form of waste to which the Waste Management Licensing Regulations 1994(22), the Special Waste Regulations 1996(23), the Hazardous Waste (Wales) Regulations 2005(24) or the Hazardous Waste (England and Wales) Regulations 2005(25) applies;
(h)intended for use as food within the meaning of section 1 of the Food Safety Act 1990(26);
(i)intended for use as feeding stuff within the meaning of section 66(1) of the Agriculture Act 1970(27);
(j)a radioactive substance or a preparation containing radioactive substances; or
(k)a medical device within the meaning of the Medical Devices Regulations 2002(28) which is invasive or used in direct contact with the human body,
in the finished state, intended for the final user.
(3) These Regulations shall not apply to—
(a)a substance or preparation which is a sample taken by an authority responsible for the enforcement of any requirement imposed by or under any enactment;
(b)a substance or preparation which is under customs control; or
(c)subject to Regulation (EC) No 689/2008 of the European Parliament and of the Council of 17 June 2008 concerning the export and import of dangerous chemicals, of which Annexes I and V are as amended from time to time, a substance or a preparation which is intended for export to a country which is not an EEA State.
(4) Regulations 6 to 11 shall only apply to substances and preparations which are supplied in packages.
(5) Regulations 6 to 11 shall not apply to munitions and explosives which are supplied with a view to obtaining an explosive or pyrotechnic effect.
(6) These Regulations shall not apply to the carriage of substances or preparations by rail, road, inland waterway, sea or air.
PART 2GENERAL REQUIREMENTS
Classification of dangerous substances and dangerous preparations
4.—(1) No person shall supply a dangerous substance or dangerous preparation unless it has been classified in accordance with paragraphs (2) to (7).
(2) The classification of a dangerous substance which is listed in Table 3.2 of part 3 of Annex VI of the CLP Regulation shall be the classification for that substance specified in that list.
(3) A dangerous substance which—
(a)is not a phase-in substance within the meaning of REACH; and
(b)is not listed in Table 3.2 of part 3 of Annex VI of the CLP Regulation; and
(c)has been registered in accordance with Title II of REACH,
shall be classified in conformity with that registration.
(4) Subject to paragraph (5), a dangerous substance which is not classified in accordance with paragraph (2) or (3) shall be classified—
(a)by ascertaining which of the properties specified in Column 2 of Schedule 1 applies to the dangerous substance and by placing the dangerous substance in one or more of the categories of danger specified in the corresponding entry in Column 1 of that Schedule;
(b)by assigning to the dangerous substance the appropriate risk phrases by the use of the criteria set out in the approved classification and labelling guide; and
(c)where it is proposed to classify a dangerous substance in the category of danger carcinogenic, mutagenic or toxic for reproduction, by an assessment of the evidence by a competent person.
(5) Before a dangerous substance is classified in accordance with paragraph (4), persons carrying out the classification shall make themselves aware of all relevant and accessible data which may exist in relation to the dangerous substance in question.
(6) Where a manufacturer, distributor or importer has classified a substance, in accordance with the provisions of paragraph (4), as a dangerous substance in the category of danger carcinogenic, mutagenic or toxic for reproduction, that person shall send to the Executive as soon as possible a document—
(a)summarising the information on which the classification was based; and
(b)including all relevant references and unpublished data,
unless that document has already been sent to the relevant authority in another EEA State in which the dangerous substance has been supplied.
(7) A dangerous preparation shall be classified in accordance with Schedule 3 and, where applicable, by use of the criteria contained in the approved classification and labelling guide.
Safety data sheets for substances and preparations
5. The requirements for safety data sheets in Article 31 of REACH shall apply as set out in that Regulation.
Packaging of dangerous substances, dangerous preparations and certain specified preparations
6.—(1) No person shall supply a dangerous substance or a dangerous preparation or a preparation specified in regulation 11(3) unless its packaging satisfies the following requirements—
(a)subject to paragraph (2), the receptacle containing the dangerous substance or dangerous preparation is designed and constructed so that its contents cannot escape;
(b)the materials constituting the packaging and fastenings are not susceptible to adverse attack by the contents or liable to form dangerous compounds with the contents;
(c)the packaging and fastenings are strong and solid throughout to ensure that they will not loosen and will meet the normal stresses and strains of handling; and
(d)any replaceable fastening fitted to the receptacle containing the dangerous substance or dangerous preparation is designed so that the receptacle can be repeatedly refastened without the contents of the receptacle escaping.
(2) Paragraph (1)(a) shall not apply where a special safety device is fitted to the receptacle.
(3) Packaging and fastenings shall be deemed to comply with the requirements of paragraphs (1)(a) to (c) if they comply with the relevant requirements of—
(a)the Carriage of Dangerous Goods and Use of Transportable Pressure Equipment Regulations 2007(29);
(b)the Merchant Shipping (Dangerous Goods and Marine Pollutants) Regulations 1997(30); or
(c)the Air Navigation (Dangerous Goods) Regulations 2002(31).
Labelling of dangerous substances and dangerous preparations
7.—(1) Subject to paragraphs (5) to (9) and regulation 8, no person shall supply a dangerous substance or dangerous preparation unless the particulars specified in paragraph (2) relating to a dangerous substance or paragraph (3) relating to a dangerous preparation are clearly shown in accordance with the requirements of regulation 10—
(a)on the receptacle containing the dangerous substance or dangerous preparation; and
(b)if that receptacle is inside one or more layers of packaging, on any such layer which is likely to be the outermost layer of packaging during the supply or use of the dangerous substance or dangerous preparation, unless such packaging permits the particulars shown on the receptacle or other packaging to be clearly seen.
(2) The particulars required under paragraph (1) in relation to a dangerous substance shall be—
(a)the name, full address and telephone number of a person in an EEA State who is responsible for supplying the substance, whether the person be its manufacturer, importer or distributor;
(b)the name of the substance being—
(i)where the substance appears in Table 3.2 of part 3 of Annex VI of the CLP Regulation, the name or one of the names listed therein for that substance; or
(ii)where the substance does not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation, an internationally recognised name; and
(c)the following particulars ascertained in accordance with Part I of Schedule 4, namely—
(i)any indications of danger together with corresponding symbols;
(ii)the risk phrases, set out in full;
(iii)the safety phrases, set out in full; and
(iv)any EC number and, in the case of a substance which is listed in Table 3.2 of part 3 of Annex VI of the CLP Regulation, the words “EC label”.
(3) The particulars required under paragraph (1) in relation to a dangerous preparation shall be—
(a)the name, full address and telephone number of a person in an EEA State who is responsible for supplying the preparation, whether that person be its manufacturer, importer or distributor;
(b)the trade name or other designation of the preparation; and
(c)the following particulars ascertained in accordance with Part I of Schedule 4, namely—
(i)identification of the constituents of the preparation which result in it being classified as a dangerous preparation,
(ii)any indications of danger together with corresponding symbols,
(iii)the risk phrases, set out in full,
(iv)the safety phrases, set out in full,
(v)in the case of a preparation intended for sale to the general public, the nominal quantity (nominal mass or nominal volume).
(4) Without prejudice to paragraph 3 of Schedule 3 to the Plant Protection Products Regulations, indications such as “non-toxic”, “non-harmful”, “non-polluting”, “ecological” or any other statement indicating that the dangerous substance or preparation is not dangerous or that is likely to lead to underestimation of the dangers of the dangerous substance or dangerous preparation shall not appear on the package.
(5) Where paragraph (6) applies, the packaging of a dangerous substance or dangerous preparation classified in one or more of the categories of danger harmful, extremely flammable, highly flammable, flammable, irritant or oxidising shall not be required to be labelled in respect of that hazardous property.
(6) This paragraph shall apply where the package contains such small quantities of that substance or preparation that there is no foreseeable risk, under conditions of supply, use and disposal, arising from that hazardous property to persons handling that substance or preparation or to other persons.
(7) The packaging of a dangerous preparation classified in the category of danger dangerous for the environment (with or without the “N” symbol) shall not be required to be labelled in respect of its environmental hazard in accordance with this regulation provided that it contains such small quantities of that preparation that there is no foreseeable risk, under conditions of supply, use and disposal, to the environment.
(8) Where the package in which a dangerous substance is supplied does not contain more than 125 millilitres of that substance the risk phrases and safety phrases need not be shown if the dangerous substance is classified only in one or more of the categories of danger—
(a)highly flammable, flammable, oxidising or irritant; or
(b)harmful, provided the dangerous substance is not sold to the general public.
(9) Where the package in which a dangerous preparation is supplied does not contain more than 125 millilitres of that preparation—
(a)the risk phrases and safety phrases need not be shown if the dangerous preparation is classified only in one or more of the categories of danger—
(i)irritant (except those assigned the risk phrase R41);
(ii)dangerous for the environment and assigned the N symbol;
(iii)oxidising; or
(iv)highly flammable; and
(b)the safety phrases need not be shown if the dangerous preparation is classified only in one or more of the categories of danger—
(i)flammable; or
(ii)dangerous for the environment and not assigned the N symbol.
(10) Where a dangerous substance or dangerous preparation is required to be labelled in accordance with these Regulations and is so labelled, that labelling shall be deemed to satisfy the requirements of—
(a)section 5 of the Petroleum (Consolidation) Act 1928(32) including that section as applied to any dangerous substance by an Order in Council made under section 19 of that Act;
(b)regulation 10 of the Dangerous Substances and Explosive Atmospheres Regulations 2002(33); and
(c)regulations 3 and 4 of the Farm and Garden Chemicals Regulations 1971(34).
Labelling of single receptacles and receptacles in outer packagings
8.—(1) Where except for this regulation a package would be required to show the particulars required by regulation 7 and to be labelled and marked in accordance with any of the national or international transport rules, it shall be sufficient compliance with regulation 7 if the package shows the particulars specified in paragraphs (2) or (3) of this regulation.
(2) Where the package consists of only a single receptacle, the specified particulars are—
(a)the particulars required by—
(i)paragraph (2)(a), (b), (c)(ii), (iii) and (iv) in the case of substances; and
(ii)paragraph (3)(a), (b), (c)(i), (iii), (iv) and (v) in the case of preparations, of regulation 7 in accordance with regulation 10;
(b)the labels and markings required by whichever of the national or international transport rules is appropriate; and
(c)where a substance or preparation has been classified as dangerous for the environment, the appropriate indication of danger and the danger symbol from Schedule 2 in accordance with regulation 10.
(3) Where the package consists of one or more receptacles in outer packagings, the particulars specified are the labels and markings required by whichever of the national or international transport rules is appropriate.
(4) For the purpose of this regulation and regulation 9(3)—
(a)the national transport rules are—
(i)the Merchant Shipping (Dangerous Goods and Marine Pollutants) Regulations 1997;
(ii)the Air Navigation (Dangerous Goods) Regulations 2002; and
(iii)the Carriage of Dangerous Goods and Use of Transportable Pressure Equipment Regulations 2007; and
(b)the international transport rules are—
(i)the European Agreement concerning the International Carriage of Dangerous Goods by Road signed at Geneva on 30th September 1957, as revised or reissued from time to time(35);
(ii)the European Agreement concerning the International Carriage of Dangerous Goods by Inland Waterway, as revised or re-issued from time to time(36);
(iii)the Technical Instructions for the Safe Transport of Dangerous Goods by Air, as revised or re-issued from time to time(37);
(iv)the International Maritime Dangerous Goods Code, as revised or re-issued from time to time(38); and
(v)the Regulation concerning the International Carriage of Dangerous Goods by Rail including its Annex which together form Appendix C to the Convention concerning the International Carriage by Rail(39), as revised or re-issued from time to time.
Particular labelling requirements for certain preparations
9.—(1) In the case of preparations to which Part II of Schedule 4 applies, the appropriate provisions of that Part shall have effect to regulate the labelling of such preparations.
(2) Subject to paragraph 3, no person shall supply a preparation to which section B or C of Part II of Schedule 4 applies unless the trade name or other designation of that preparation and the name, full address and telephone number of a person in an EEA state who is responsible for supplying that preparation (whether the person is its manufacturer, importer or distributor) are clearly shown in accordance with the requirements of regulation 10—
(a)on the receptacle containing that preparation; and
(b)if that receptacle is inside one or more layers of packaging, on any such layer which is likely to be the outermost layer of packaging during the supply or use of that preparation, unless such packaging permits the particulars shown on the receptacle or other packaging to be clearly seen.
(3) Where a package would be required to be labelled and marked in accordance with any of the national or international transport rules listed in regulation 8(4) and the package consists of one or more receptacles in outer packagings, it shall be sufficient compliance with paragraph (2) if the package shows the labels and markings required by whichever of the national or international rules is appropriate.
(4) The supplier of an aerosol dispenser which contains a dangerous substance or dangerous preparation which has been classified in the category of danger “flammable”, “highly flammable” or “extremely flammable” may choose to omit from the label—
(a)in the case of a dangerous substance, the particulars referred to in paragraphs (i) to (iii) of regulation 7(2)(c) which relate to that category of danger; and
(b)in the case of a dangerous preparation, the particulars referred to in paragraphs (ii) to (iv) of regulation 7(3)(c) which relate to that category of danger,
provided the conditions specified in (5) are satisfied.
(5) The conditions referred to in paragraph (4) are that the supplier—
(a)is in possession of evidence which shows that the contents of the aerosol dispenser do not present a risk of ignition under normal or reasonably foreseeable conditions of use; and
(b)identifies the quantity of flammable material contained in the aerosol dispenser in the form of the following inscription on the label—
“X% by mass of the contents are flammable”.
(6) In the case of a plant protection product approved under the Plant Protection Products Regulations or a product approved under the Control of Pesticides Regulations 1986(40), the labelling information required by these Regulations shall be accompanied by the wording “To avoid risks to man and the environment, comply with the instructions for use”.
Methods of marking or labelling packages
10.—(1) Any package which is required to be labelled in accordance with regulations 7 to 9 may carry the particulars required to be on the label clearly and indelibly marked on a part of that package reserved for that purpose and, unless the context otherwise requires, any reference in these Regulations to a label includes a reference to that part of the package so reserved.
(2) Subject to paragraph (7), any label required to be carried on a package shall be securely fixed to the package with its entire surface in contact with it and the label shall be clearly and indelibly printed.
(3) The colour and nature of the marking shall be such that any symbol and the wording stand out clearly from the background and the wording shall be of such size and spacing as to be easily read.
(4) The package shall be so labelled that the particulars can be read horizontally when the package is set down normally.
(5) Subject to paragraph (7), the dimensions of the label required by regulation 7 shall be as follows—
| Capacity of package | Dimensions of label |
|---|---|
(a)not exceeding 3 litres | if possible at least 52 x 74 millimetres |
(b)exceeding 3 litres but not exceeding 50 litres | at least 74 x 105 millimetres |
(c)exceeding 50 litres but not exceeding 500 litres | at least 105 x 148 millimetres |
(d)exceeding 500 litres | at least 148 x 210 millimetres |
(6) A symbol required to be shown in accordance with regulation 7(2)(c)(i) or 7(3)(c)(ii) and specified in column 3 of Schedule 2 shall be printed in black on an orange-yellow background and its size (including the orange-yellow background) shall be at least equal to an area of one-tenth of that of a label which complies with paragraph (5) and shall not in any case be less than 100 square millimetres.
(7) If the package is an awkward shape or so small that it is unsuitable to attach a label complying with paragraphs (2) and (5), the label shall be attached in some other appropriate manner.
Child resistant fastenings, tactile warning devices and other consumer protection measures
11.—(1) The British and International Standards referred to in this regulation are further described in Schedule 5.
(2) Subject to paragraphs (4) and (5), no person shall supply to the general public a substance or a preparation specified in paragraph (3) in a receptacle of any size fitted with—
(a)a replaceable closure unless the packaging complies with the requirements of BS EN 28317; or ISO 8317; or
(b)a non-replaceable closure unless the packaging complies with the requirements of EN 862.
(3) The substances and preparations referred to in paragraph (2) are—
(a)dangerous substances and dangerous preparations which are required to be labelled with the indication of danger “very toxic”, “toxic” or “corrosive”;
(b)preparations containing methanol in a concentration equal to or more than 3% by weight;
(c)preparations containing dichloromethane in a concentration equal to or more than 1% by weight;
(d)substances which are assigned the risk phrase R65 in Table 3.2 of part 3 of Annex VI of the CLP Regulation, except where such a substance is supplied in an aerosol dispenser or a container fitted with a sealed spray attachment; and
(e)substances and preparations which are assigned the risk phrase R65 and are classified and labelled according to the approved classification and labelling guide, except where such a substance or preparation is supplied in an aerosol dispenser or a container fitted with a sealed spray attachment.
(4) Paragraph (2) shall not apply if the person supplying the substance or preparation in question can show that it is obvious that the packaging in which the substance or preparation is supplied is sufficiently safe for children because they cannot obtain access to the contents without the help of a tool.
(5) If the packaging, in which a substance or preparation specified in paragraph (3) is supplied to the general public, was approved on or before 31 May 1993 by the British Standards Institution as complying with the requirements of BS 6652, that packaging shall be deemed to comply with the requirements of BS EN 28317.
(6) No person shall supply a dangerous preparation or a preparation specified in paragraph (3) to the general public if the packaging in which that preparation is supplied has—
(a)either a shape or a designation or both likely to attract or arouse the active curiosity of children or to mislead consumers; or
(b)either a presentation or a designation or both used for—
(i)human or animal foodstuffs;
(ii)medicinal products; or
(iii)cosmetic products.
(7) Subject to paragraph (9), no person shall supply to the general public a dangerous substance or a dangerous preparation specified in paragraph (8) in a receptacle of any size, unless the packaging in which that dangerous substance or dangerous preparation is supplied carries a tactile warning of danger in accordance with EN ISO 11683.
(8) The dangerous substances and the dangerous preparations referred to in paragraph (7) are those which are required to be labelled with one or more of the following indications of danger, namely—
(a)very toxic;
(b)toxic;
(c)corrosive;
(d)harmful;
(e)extremely flammable; or
(f)highly flammable.
(9) Paragraph (7) shall not apply to an aerosol dispenser which is classified and labelled only with the indication of danger extremely flammable or highly flammable.
(10) For the purpose of ascertaining whether there has been a contravention of paragraph (2), a duly authorised officer of the enforcing authority may require the person supplying a substance or a preparation to which that paragraph applies to provide the duly authorised officer with a certificate from a qualified test house stating that—
(a)the closure is such that it is not necessary to test to BS EN 28317 or ISO 8317; or
(b)the closure has been tested and found to conform to BS EN 28317 or ISO 8317.
(11) In this regulation, “qualified test house” means a laboratory that conforms to BS 7501 or EN 45 000.
Retention of data for dangerous preparations
12.—(1) The person who is responsible for first supplying a dangerous preparation shall maintain a record of the information—
(a)used for the purposes of classifying that dangerous preparation in accordance with regulation 4;
(b)used for the purposes of labelling that dangerous preparation in accordance with regulation 7; and
(c)relating to any child resistant fastening or any tactile warning which forms part of the packaging in which the dangerous preparation in question is contained in accordance with regulation 11,
for at least three years after the date on which that dangerous preparation was supplied by that person for the last time.
(2) When requested by the enforcing authority to do so, a person referred to in paragraph (1) shall make the record, or a copy of the record, maintained by the person in accordance with that paragraph, available to the enforcing authority within 28 days of the date of the request.
(3) When requested to do so by the enforcing authority, a person who supplies a dangerous preparation shall provide to the enforcing authority a copy of any certificate issued by a qualified test house.
Transitional provisions for dangerous substances, dangerous preparations and certain specified preparations
13.—(1) Regulation 4 shall not have effect on or after 1 June 2015.
(2) Where a substance has been classified, labelled and packaged in accordance with the CLP Regulation, regulations 6 to 11 shall not apply to that substance.
(3) Where a preparation has been classified, labelled and packaged in accordance with the CLP Regulation, regulations 6 to 11 shall not apply to that preparation.
(4) Insofar as they relate to substances, regulations 6 to 11 shall not have effect on or after 1 December 2010.
(5) Insofar as they relate to preparations, regulations 6 to 11 shall not have effect on or after 1 June 2015.
(6) Regulation 12 shall not apply on or after 1 June 2018.
PART 3MISCELLANEOUS
Enforcement
14.—(1) To the extent that they would not otherwise do so, sections—
(a)16 to 28 (approval of codes of practice; enforcement; indemnification of inspectors; power to obtain information and restrictions on disclosure of information);
(b)33 to 42 (provisions as to offences); and
(c)47(2) (civil liability),
of the 1974 Act shall apply to these Regulations and the CLP Regulation as if these Regulations and the CLP Regulation were health and safety Regulations for the purposes of that Act except that those sections shall not apply to duties placed by the CLP Regulation on the competent authority or the Member State.
(2) Any function of the Health and Safety Executive under any other provision of the 1974 Act under or in respect of health and safety Regulations (including their enforcement) shall be exercisable as if these Regulations and the CLP Regulation were health and safety Regulations for the purposes of that Act to the extent that they would not otherwise do so.
(3) Notwithstanding regulation 3 of the Health and Safety (Enforcing Authority) Regulations 1998(41) and subject to paragraphs (4) and (5), the enforcing authority for these Regulations and the CLP Regulation shall be the Executive.
(4) Subject to paragraph (5), where a substance or preparation is supplied, or a substance, mixture or article falling within the meaning of and the provisions of the CLP Regulation is placed on the market within the meaning of the CLP Regulation in or from premises which are registered under section 75 of the Medicines Act 1968(42), the enforcing authority shall be the Royal Pharmaceutical Society.
(5) The enforcing authority for these Regulations and the CLP Regulation shall be the local weights and measures authority—
(a)where a substance or preparation is supplied or a substance, mixture or article falling within the meaning of and the provisions of the CLP Regulation is placed on the market within the meaning of the CLP Regulation other than in the circumstances referred to in paragraph (4)—
(i)in or from any shop, mobile vehicle, market stall or other retail outlet, or
(ii)otherwise to members of the public, including by way of free sample, prize or mail order;
(b)for regulation 11;
(c)for Articles 35(2) and 48 of the CLP Regulation.
(6) In every case where, by virtue of this regulation and the CLP Regulation, these Regulations and the CLP Regulation are enforced by the Royal Pharmaceutical Society or the local weights and measures authority, they shall be enforced as if they were safety regulations made under section 11 of the Consumer Protection Act 1987(43) and the provisions of section 12 of that Act shall apply to these Regulations and the CLP Regulation as if they were safety regulations for the purposes of that Act and as if the maximum period of imprisonment on summary conviction specified in subsection (5) thereof were 3 months instead of 6 months.
Defence
15. In any proceedings for an offence for a contravention of any of the provisions of these regulations and the CLP Regulation it shall be a defence for the person charged to prove that the person took all reasonable precautions and exercised all due diligence to avoid the commission of the offence.
Extension outside Great Britain
16. These Regulations and the CLP Regulation shall apply to any activity outside Great Britain to which sections 1 to 59 and 80 to 82 of the 1974 Act apply by virtue of the Health and Safety at Work etc. Act 1974 (Application outside Great Britain) Order 2001(44) as they apply to activities within Great Britain.
Revocations and amendments
17. The Regulations specified in the Table in Schedule 6 are amended in accordance with the provisions of that Table.
18. The Regulations specified in the Table in Schedule 7 are revoked to the extent specified in that Table.
Signed by authority of the Secretary of State for Work and Pensions.
William D. McKenzie
Parliamentary Under-Secretary of State,
Department for Work and Pensions
16th March 2009
Regulations 2(1) and 4(4)
SCHEDULE 1CLASSIFICATION OF DANGEROUS SUBSTANCES AND DANGEROUS PREPARATIONS
categories of danger
| Column 1 | Column 2 | Column 3 |
|---|---|---|
| Category of danger | Property (See Note 1) | Symbol-letter |
Notes 1. As further described in the approved classification and labelling guide. 2. The categories are specified in the approved classification and labelling guide. 3. In certain cases specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation and in the approved classification and labelling guide substances and preparations classified as dangerous for the environment do not require to be labelled with the symbol and indication of danger. | ||
| PHYSICO-CHEMICAL PROPERTIES | ||
| Explosive | Solid, liquid, pasty or gelatinous substances and preparations which may react exothermically without atmospheric oxygen thereby quickly evolving gases, and which under defined test conditions detonate, quickly deflagrate or upon heating explode when partially confined. | E |
| Oxidising | Substances and preparations which give rise to a highly exothermic reaction in contact with other substances, particularly flammable substances. | O |
| Extremely flammable | Liquid substances and preparations having an extremely low flash point and a low boiling point and gaseous substances and preparations which are flammable in contact with air at ambient temperature and pressure. | F+ |
| Highly flammable | The following substances and preparations, namely— (a) substances and preparations which may become hot and finally catch fire in contact with air at ambient temperature without any application of energy, (b) solid substances and preparations which may readily catch fire after brief contact with a source of ignition and which continue to burn or to be consumed after removal of the source of ignition, (c) liquid substances and preparations having a very low flash point, or (d) substances and preparations which, in contact with water or damp air, evolve extremely flammable gases in dangerous quantities. | F |
| Flammable | Liquid substances and preparations having a low flash point. | none |
| HEALTH EFFECTS | ||
| Very toxic | Substances and preparations which in very low quantities cause death or acute or chronic damage to health when inhaled, swallowed or absorbed via the skin. | T+ |
| Toxic | Substances and preparations which in low quantities cause death or acute or chronic damage to health when inhaled, swallowed or absorbed via the skin. | T |
| Harmful | Substances and preparations which may cause death or acute or chronic damage to health when inhaled, swallowed or absorbed via the skin. | Xn |
| Corrosive | Substances and preparations which may, on contact with living tissues, destroy them. | C |
| Irritant | Non-corrosive substances and preparations which, through immediate, prolonged or repeated contact with the skin or mucous membrane, may cause inflammation. | Xi |
| Sensitising | Substances and preparations which, if they are inhaled or if they penetrate the skin, are capable of eliciting a reaction by hypersensitization such that on further exposure to the substance or preparation, characteristic adverse effects are produced. | |
| Sensitising by inhalation | Xn | |
| Sensitising by skin contact | Xi | |
| Carcinogenic (SeeNote 2) | Substances and preparations which, if they are inhaled or ingested or if they penetrate the skin, may induce cancer or increase its incidence. | |
| Category 1 | T | |
| Category 2 | T | |
| Category 3 | Xn | |
| Mutagenic (See Note 2) | Substances and preparations which, if they are inhaled or ingested or if they penetrate the skin, may induce heritable genetic defects or increase their incidence. | |
| Category 1 | T | |
| Category 2 | T | |
| Category 3 | Xn | |
| Toxic for reproduction (see Note 2) | Substances and preparations which, if they are inhaled or ingested or if they penetrate the skin, may produce or increase the incidence of non-heritable adverse effects in the progeny and/or of male or female reproductive functions or capacity. | |
| Category 1 | T | |
| Category 2 | T | |
| Category 3 | Xn | |
| ENVIRONMENTAL EFFECTS | ||
| Dangerous for the environment (SeeNote 3) | Substances and preparations which, were they to enter into the environment, would present or might present an immediate or delayed danger for one or more components of the environment. | N |
Regulations 2(1), 8(2) and 10(6)
SCHEDULE 2INDICATIONS OF DANGER AND SYMBOLS FOR DANGEROUS SUBSTANCES AND DANGEROUS PREPARATIONS
Regulation 4(7)
SCHEDULE 3PROVISIONS FOR CLASSIFYING DANGEROUS PREPARATIONS
PART 1GENERAL PROVISIONS
Application
1. The provisions of this Schedule shall apply for the classification of preparations.
Interpretation and application
2.—(1) In this Schedule, for the purposes of classification—
“physico-chemical properties” means the properties to be applied for the classifications “explosive”, “oxidising”, “extremely flammable”, “highly flammable” or “flammable”;
“health effects” means the effects to be assessed for the classifications “very toxic”, “toxic”, “harmful”, “corrosive”, “irritant”, “sensitising”, “carcinogenic”, “mutagenic” or “toxic for reproduction”; and
“environmental hazards” means the hazards to be assessed for the classification “dangerous for the environment”.
(2) In its application to preparations that are gases, this Part shall be modified so that reference to concentrations expressed as percentage by weight are to concentrations expressed as the same percentage by volume.
Classification of preparations by physico-chemical properties
3.—(1) The requisite physico-chemical properties for the classification of preparations shall be determined in accordance with the criteria set out in the approved classification and labelling guide.
(2) Subject to sub-paragraph (3), preparations shall be classified as explosive, oxidising, extremely flammable, highly flammable or flammable when they satisfy the criteria referred to in sub-paragraph (1) for the category of danger.
(3) The determination of explosive, oxidising, extremely flammable, highly flammable or flammable properties is not necessary provided that—
(a)none of the constituents possess such properties and that, on the basis of information available to the manufacturer, the preparation is unlikely to present dangers of this kind;
(b)in the event of a change in composition of a preparation of known composition, scientific evidence indicates that a reassessment of the hazards will not lead to a change in classification; and
(c)in the case of a preparation supplied in the form of an aerosol, that preparation satisfies the provisions of article 8.1a of Council Directive 75/324/EEC(45).
Classification of preparations by health effects
4.—(1) The health effects of a preparation shall be assessed by one or more of the following methods—
(a)by the conventional method described in paragraphs 7 to 15 using concentration limits; or
(b)by the criteria set out in the approved classification and labelling guide in relation to the preparation for an appropriate classification and label.
(2) Any one or more of the health effects of the preparation which are not assessed by the method set out in sub-paragraph (1)(b) shall be assessed in accordance with the conventional method.
(3) Where the health effects have been established by both methods, the results of the method referred to in sub-paragraph (1)(b) shall be used for classifying the preparation except in the case of carcinogenic and mutagenic effects and toxic effects for reproduction, when the conventional method referred to in sub-paragraph (1)(a) shall always be used.
(4) Where it can be demonstrated—
(a)by epidemiological studies, by scientifically valid case studies as specified in the approved classification and labelling guide or by statistically backed experience (such as the assessment of data from poison information units or concerning occupational diseases) that toxicological effects on man differ from those suggested by the application of the methods set out in paragraph (1), then the preparation shall be classified according to its effects on man;
(b)that owing to effects such as potentiation, a conventional assessment would underestimate the toxicological hazard, those effects shall be taken into account in classifying the preparation; or
(c)that owing to effects such as antagonism, a conventional assessment would overestimate the toxicological hazard, those effects shall be taken into account in classifying the preparation.
(5) Subject to sub-paragraph (6), for preparations of a known composition, with the exception of plant protection products, classified in accordance with the method referred to in sub-paragraph (1)(b), a new health effect assessment shall be performed either by the method referred to in sub-paragraph (1)(a) or (1)(b) whenever—
(a)changes of composition of the initial concentration, as a weight/weight or volume/volume percentage, of one or more of the dangerous constituents are introduced by the manufacturer which exceed the permitted variations set out in the following table—
| Initial concentration range of the constituent | Permitted variation in actual concentration of the constituent | |
|---|---|---|
| ≤2.5% | ±30% | |
| >2.5 | ≤10% | ±20% |
| >10 | ≤25% | ±10% |
| >25 | ≤100% | ±5 |
or,
(b)changes of composition involving the substitution or addition of one or more constituents, which may or may not be dangerous within the definitions in Schedule 1, are introduced by the manufacturer.
(6) The revised assessment required by sub-paragraph (5) shall not be required where there is a valid scientific justification for considering that a re-evaluation of the hazard will not result in a change of classification.
Use of concentration limits in classification for health effects by the conventional method
5.—(1) In accordance with paragraph 4(1)(a), the health effects shall be assessed by the conventional method described in paragraphs 7 to 15 using concentration limits.
(2) Where the substances concerned are dangerous substances and are listed as dangerous substances in Table 3.2 of part 3 of Annex VI of the CLP Regulation and are assigned concentration limits necessary for the application of the method of assessment described below, these concentration limits shall be used.
(3) Where the substances concerned are dangerous substances and do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation as dangerous substances or appear there without the concentration limits necessary for the application of the method of evaluation described below, the concentration limits shall be assigned in accordance with Part II of this Schedule.
Lower limits of concentration
6.—(1) For preparations to which this Schedule applies, account shall be taken of dangerous substances which are classified as dangerous on the basis of their health or environmental effects (whether they are present as additives or impurities) when their concentrations are equal to or greater than those defined in the following table unless lower limits are given in Table 3.2 of part 3 of Annex VI of the CLP Regulation or in Part II of this Schedule—
| Category of danger of the substance | Concentration to take into consideration for | |
|---|---|---|
| gaseous preparations % vol/vol | other preparations % w/w | |
| Very toxic | ≥0.02 | ≥0.1 |
Carcinogenic Category 1 or 2 | ≥0.02 | ≥0.1 |
Mutagenic Category 1 or 2 | ≥0.02 | ≥0.1 |
Toxic for reproduction Category 1 or 2 | ≥0.02 | ≥0.1 |
| Harmful | ≥0.2 | ≥1 |
| Corrosive | ≥0.02 | ≥1 |
| Irritant | ≥0.2 | ≥1 |
| Sensitising | ≥0.2 | ≥1 |
Carcinogenic Category 3 | ≥0.2 | ≥1 |
Mutagenic Category 3 | ≥0.2 | ≥1 |
Toxic for reproduction Category 3 | ≥0.2 | ≥1 |
| Dangerous for the environment N | ≥0.1 | |
Dangerous for the environment Ozone | ≥0.1 | ≥0.1 |
| Dangerous for the environment | ≥1 | |
(2) Some substances may have more than one health effect and each of these properties shall be characterised by its specific concentration limit.
Classification by the conventional method as very toxic
7.—(1) The following preparations shall be classified as very toxic owing to their acute lethal effects and assigned the symbol “T+”, the indication of danger “very toxic” and the risk phrase R26, R27 or R28—
(a)preparations containing one or more substances classified as very toxic that produce such effects, in individual concentrations equal to or exceeding—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in paragraph 1 of Part II of this Schedule (Table I or Table IA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits;
(b)preparations containing more than one substance classified as very toxic in lower individual concentrations than the limits specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation or in paragraph 1 of Part II of this Schedule (Table I or Table IA), if the sum of the quotients obtained by dividing the percentage weight of each very toxic substance in the preparation by the very toxic limit specified for that substance is 1 or more, i.e.—
where—
PT+ is the percentage by weight of each very toxic substance in the preparation,
LT+ is the very toxic limit specified for each very toxic substance expressed as a percentage by weight or by volume.
(2) The following preparations shall be classified as very toxic owing to their non-lethal irreversible effects after a single exposure and assigned the symbol “T+”, the indication of danger “very toxic” and the risk phrase R39/route of exposure—
Preparations containing one or more dangerous substances which produce such effects in individual concentrations equal to or exceeding—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in paragraph 2 of Part II of this Schedule (Table II or Table IIA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits.
Classification by the conventional method as toxic
8.—(1) The following preparations shall be classified as toxic owing to their acute lethal effects and assigned the symbol “T”, the indication of danger “toxic” and the risk phrase R23, R24, or R25—
(a)preparations containing one or more substances classified as very toxic or toxic that produce such effects in individual concentrations equal to or exceeding—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in paragraph 1 of Part II of this Schedule (Table I or Table IA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits;
(b)preparations containing more than one substance classified as very toxic or toxic in lower individual concentrations than the limits specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation or in paragraph 1 of Part II of this Schedule (Table I or Table IA) if the sum of the quotients obtained by dividing the percentage weight of each very toxic or toxic substance in the preparation by the toxic limit specified for that substance is 1 or more, i.e.—
where—
PT+ is the percentage by weight or by volume of each very toxic substance in the preparation,
PT is the percentage by weight or by volume of each toxic substance in the preparation,
LT is the respective toxic limit specified for each very toxic or toxic substance expressed as a percentage by weight or by volume.
(2) The following preparations shall be classified as toxic owing to their non-lethal irreversible effects after a single exposure and assigned the symbol “T”, the indication of danger “toxic” and the risk phrase R39/route of exposure—
Preparations containing one or more dangerous substances classified as very toxic or toxic which produce such effects in individual concentrations equal to or exceeding—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in paragraph 2 of Part II of this Schedule (Table II or Table IIA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits.
(3) The following preparations shall be classified as toxic owing to their long-term effects and assigned the symbol “T”, the indication of danger “toxic” and the risk phrase R48/route of exposure—
Preparations containing one or more dangerous substances which produce such effects in individual concentrations equal to or exceeding—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in paragraph 3 of Part II of this Schedule (Table III or Table IIIA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits.
Classification by the conventional method as harmful
9.—(1) The following preparations shall be classified as harmful owing to their acute lethal effects and assigned the symbol “Xn”, the indication of danger “harmful” and the risk phrase R20, R21 or R22—
(a)preparations containing one or more substances classified as very toxic, toxic or harmful and that produce such effects in individual concentrations equal to or exceeding—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in paragraph 1 of Part II of this Schedule (Table I or Table IA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits;
(b)preparations containing more than one substance classified as very toxic, toxic or harmful in lower individual concentrations than the limits specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation or in paragraph 1 of Part II of this Schedule (Table I or Table IA) if the sum of the quotients obtained by dividing the percentage weight of each very toxic, toxic or harmful substance in the preparation by the harmful limit specified for that substance is 1 or more, i.e.—
where—
PT+ is the percentage by weight or by volume of each very toxic substance in the preparation,
PT is the percentage by weight or by volume of each toxic substance in the preparation,
PXn is the percentage by weight or by volume of each harmful substance in the preparation,
LXn is the respective harmful limit specified for each very toxic, toxic or harmful substance expressed as a percentage by weight or by volume.
(2) The following preparations shall be classified as harmful owing to their acute effects to the lungs if swallowed and assigned the symbol “Xn”, the indication of danger “harmful” and the risk phrase R65—Preparations classified as harmful according to the criteria specified in the approved classification and labelling guide.
In applying the conventional method according to sub-paragraph (1), no account shall be taken of the classification of a substance as R65.
(3) The following preparations shall be classified as harmful owing to their non-lethal irreversible effects after a single exposure and assigned the symbol “Xn”, the indication of danger “harmful” and the risk phrase R68/route of exposure—
Preparations containing one or more dangerous substances classified as very toxic, toxic or harmful which produce such effects in individual concentrations equal to or exceeding—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in paragraph 2 of Part II of this Schedule (Table II or Table IIA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits.
(4) The following preparations shall be classified as harmful owing to their long-term effects and assigned the symbol “Xn”, the indication of danger “harmful” and the risk phrase R48/ route of exposure—
Preparations containing one or more dangerous substances classified as toxic or harmful that produce such effects in individual concentrations equal to or exceeding—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in paragraph 3 of Part II of this Schedule (Table III or Table IIIA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits.
Classification by the conventional method as corrosive
10.—(1) The following preparations shall be classified as corrosive and assigned the symbol “C”, the indication of danger “corrosive” and the risk phrase R35—
(a)preparations containing one or more substances classified as corrosive to which is assigned the risk phrase R35 in individual concentrations equal to or exceeding—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in paragraph 4 of Part II of this Schedule (Table IV or Table IVA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits;
(b)preparations containing more than one substance classified as corrosive to which is assigned the risk phrase R35 in lower individual concentrations than the limits specified either in Table 3.2 of part 3 of Annex VI of the CLP Regulation or in paragraph 4 of Part II of this Schedule (Table IV or Table IVA) if the sum of the quotients obtained by dividing the percentage weight of each corrosive substance in the preparation by the corrosive limit R35 specified for that substance is 1 or more, ie—
where—
PC. R35 is the percentage by weight or by volume of each corrosive substance to which is assigned the risk phrase R35 in the preparation,
LC. R35 is the corrosive limit R35 specified for each corrosive substance to which is assigned the risk phrase R35 expressed as a percentage by weight or by volume.
(2) The following preparations shall be classified as corrosive and assigned the symbol “C”, the indication of danger “corrosive” and the risk phrase R34—
(a)preparations containing one or more substances classified as corrosive to which is assigned the risk phrase R35 or R34 in individual concentrations equal to or exceeding—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in paragraph 4 of Part II of this Schedule (Table IV or Table IVA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits;
(b)preparations containing more than one substance classified as corrosive to which is assigned the risk phrase R35 or R34 in lower individual concentrations than the limits specified either in Table 3.2 of part 3 of Annex VI of the CLP Regulation or in paragraph 4 of Part II of this Schedule (Table IV or Table IVA) if the sum of the quotients obtained by dividing the percentage weight of each corrosive substance in the preparation by the corrosive limit R34 specified for that substance is 1 or more, ie—
where—
PC. R35 is the percentage by weight or by volume of each corrosive substance to which is assigned the risk phrase R35 in the preparation,
PC. R34 is the percentage by weight or by volume of each corrosive substance to which is assigned the risk phrase R34 in the preparation,
LC. R34 is the respective corrosive limit R34 specified for each corrosive substance to which is assigned the risk phrase R35 or R34 expressed as a percentage by weight or by volume.
Classification by the conventional method as irritant
11.—(1) The following preparations shall be classified as irritants liable to cause serious eye damage and assigned the symbol “Xi”, the indication of danger “irritant” and risk phrase R41—
(a)preparations containing one or more substances classified as irritant to which is assigned the risk phrase R41 in individual concentrations equal to or exceeding—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in paragraph 4 of Part II of this Schedule (Table IV or Table IVA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits;
(b)preparations containing more than one substance classified as irritant to which is assigned the risk phrase R41, or classified as corrosive and to which is assigned the risk phrase R35 or R34, in lower individual concentrations than the limits specified either in Table 3.2 of part 3 of Annex VI of the CLP Regulation or in paragraph 4 of Part II of this Schedule (Table IV or Table IVA) if the sum of the quotients obtained by dividing the percentage weight of each irritant substance in the preparation by the irritant limit R41 specified for that substance is 1 or more, ie—
where—
PC. R35 is the percentage by weight or by volume of each corrosive substance to which is assigned the risk phrase R35 in the preparation,
PC. R34 is the percentage by weight or by volume of each corrosive substance to which is assigned the risk phrase R34 in the preparation,
PXi. R41 is the percentage by weight or by volume of each irritant substance to which is assigned the risk phrase R41 in the preparation,
LXi. R41 is the respective irritant limit R41 specified for each corrosive substance to which is assigned the risk phrase R35 or R34 or irritant substance to which is assigned the risk phrase R41, expressed as a percentage by weight or by volume.
(2) The following preparations shall be classified as irritant to eyes and assigned the symbol “Xi”, the indication of danger “irritant” and risk phrase R36—
(a)preparations containing one or more substances classified as corrosive to which is assigned the risk phrase R35 or R34 or as irritant to which is assigned the risk phrase R41 or R36 in individual concentrations equal to or exceeding—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in paragraph 4 of Part II of this Schedule (Table IV or Table IVA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits;
(b)preparations containing more than one substance classified as irritant to which is assigned the risk phrase R41 or R36 or as corrosive and to which is assigned the risk phrase R35 or R34, in lower individual concentrations than the limits specified either in Table 3.2 of part 3 of Annex VI of the CLP Regulation or in paragraph 4 of Part II of this Schedule (Table IV or Table IVA) if the sum of the quotients obtained by dividing the percentage weight of each irritant substance in the preparation by the irritant limit R36 specified for that substance is 1 or more, ie—
where—
PC. R35 is the percentage by weight or by volume of each corrosive substance to which is assigned the risk phrase R35 in the preparation,
PC. R34 is the percentage by weight or by volume of each corrosive substance to which is assigned the risk phrase R34 in the preparation,
PXi. R41 is the percentage by weight or by volume of each irritant substance to which is assigned the risk phrase R41 in the preparation,
PXi. R36 is the percentage by weight or by volume of each irritant substance to which is assigned the risk phrase R36 in the preparation,
LXi. R36 is the respective irritant limit R36 specified for each corrosive substance to which is assigned the risk phrase R35 or R34 or irritant substance to which is assigned the risk phrase R41 or R36, expressed as a percentage by weight or by volume.
(3) The following preparations shall be classified as irritant to skin and assigned the symbol “Xi”, the indication of danger “irritant” and the risk phrase R38—
(a)preparations containing one or more substances classified as irritant and to which is assigned the risk phrase R38 or as corrosive and to which is assigned the risk phrase R35 or R34, in individual concentrations equal to or exceeding—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in paragraph 4 of Part II of this Schedule (Table IV or Table IVA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits;
(b)preparations containing more than one substance classified as irritant to which is assigned the risk phrase R38, or as corrosive and to which is assigned the risk phrase R35 or R34 in lower individual concentrations than the limits specified either in Table 3.2 of part 3 of Annex VI of the CLP Regulation or in paragraph 4 of Part II of this Schedule (Table IV or Table IVA) if the sum of the quotients obtained by dividing the percentage weight of each substance in the preparation by the irritant limit R38 specified for that substance is 1 or more, ie—
where—
PC. R35 is the percentage by weight or by volume of each corrosive substance to which is assigned the risk phrase R35 in the preparation,
PC. R34 is the percentage by weight or by volume of each corrosive substance to which is assigned the risk phrase R34 in the preparation,
PXi. R38 is the percentage by weight or by volume of each irritant substance to which is assigned the risk phrase R38 in the preparation,
LXi. R38 is the respective irritant limit R38 specified for each corrosive substance to which is assigned the risk phrase R35 or R34 or irritant substance to which is assigned the risk phrase R38, expressed as a percentage by weight or by volume.
(4) The following preparations shall be classified as irritant to the respiratory system and assigned the symbol “Xi”, the indication of danger “irritant” and risk phrase R37—
(a)preparations containing one or more substances classified as irritant to which is assigned the risk phrase R37 in individual concentrations equal to or exceeding—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in paragraph 4 of Part II of this Schedule (Table IV or Table IVA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits;
(b)preparations containing more than one substance classified as irritant to which is assigned the risk phrase R37 in lower individual concentrations than the limits specified either in Table 3.2 of part 3 of Annex VI of the CLP Regulation or in paragraph 4 of Part II of this Schedule (Table IV or Table IVA) if the sum of the quotients obtained by dividing the percentage weight of each irritant substance in the preparation by the irritant limit R37 specified for that substance is 1 or more, ie—
where—
PXi. R37 is the percentage by weight or by volume of each irritant substance to which is assigned the risk phrase R37 in the preparation,
LXi. R37 is the irritant limit R37 specified for each irritant substance to which is assigned the risk phrase R37, expressed as a percentage by weight or by volume.
(c)gaseous preparations containing more than one substance classified as irritant and to which is assigned the risk phrase R37 or as corrosive and to which is assigned the risk phrase R35 or R34 in lower individual concentrations than the limits specified either in Table 3.2 of part 3 of Annex VI of the CLP Regulation or in paragraph 4 of Part II of this Schedule (Table IV or Table IVA) if the sum of the quotients obtained by dividing the percentage volume of each substance in the preparation by the irritant limit R37 specified for that substance is 1 or more, ie—
where—
PC. R35 is the percentage by volume of each corrosive substance to which is assigned the risk phrase R35 in the preparation,
PC. R34 is the percentage by volume of each corrosive substance to which is assigned the risk phrase R34 in the preparation,
PXi. R37 is the percentage by volume of each irritant substance to which is assigned the risk phrase R37 in the preparation,
LXi. R37 is the respective irritant limit R37 specified for each gaseous corrosive substance to which is assigned the risk phrase R35 or R34 or gaseous irritant substance to which is assigned the risk phrase R37, expressed as a percentage by weight or by volume.
Classification by the conventional method as sensitising
12.—(1) The following preparations shall be classified as sensitising by skin contact and assigned the symbol “Xi”, the indication of danger “irritant” and the risk phrase R43—
Preparations containing one or more substances classified as sensitising and to which is assigned the risk phrase R43 that produces such effects in individual concentrations equal to or exceeding—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in paragraph 5 of Part II of this Schedule (Table V or Table VA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits.
(2) The following preparations shall be classified as sensitising by inhalation and assigned the symbol “Xn”, the indication of danger “harmful” and the risk phrase R42—
Preparations containing one or more substances classified as sensitising and to which is assigned risk phrase R42 that produces such effects in individual concentrations equal to or exceeding—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in paragraph 5 of Part II of this Schedule (Table V or Table VA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits.
Classification by the conventional method as carcinogenic
13.—(1) Preparations shall be classified as carcinogenic category 1 or 2 and assigned the symbol “T” and the risk phrase R45 or R49 if they contain one or more substances producing such effects to which is assigned the risk phrase R45 or R49 which denotes carcinogenic substances in category 1 and category 2 in individual concentrations equal to or exceeding—
(a)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(b)the concentration specified in paragraph 6 of Part II of this Schedule (Table V or Table VIA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits.
(2) Preparations shall be classified as carcinogenic category 3 and assigned the symbol “Xn” and risk phrase R40 if they contain one or more substances producing such effects to which is assigned the risk phrase R40 which denotes carcinogenic substances in category 3 in individual concentrations equal to or exceeding—
(a)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(b)the concentration specified in paragraph 6 of Part II of this Schedule (Table VI or Table VIA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits.
Classification by the conventional method as mutagenic
14.—(1) Preparations shall be classified as mutagenic category 1 or 2 and assigned the symbol “T” and risk phrase R46 if they contain one or more substances producing such effects to which is assigned the risk phrase R46 which denotes mutagenic substances in category 1 and category 2 in individual concentrations equal to or exceeding—
(a)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(b)the concentration specified in paragraph 6 of Part II of this Schedule (Table VI or Table VIA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits.
(2) Preparations shall be classified as mutagenic category 3 and assigned the symbol “Xn” and the risk phrase R68 if they contain one or more substances producing such effects to which is assigned the risk phrase R68 which denotes mutagenic substances in category 2 in individual concentrations equal to or exceeding—
(a)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(b)the concentration specified in paragraph 6 of Part II of this Schedule (Table VI or Table VIA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits.
Classification by the conventional method as toxic for reproduction
15.—(1) Preparations shall be classified as toxic for reproduction category 1 or 2 and assigned the symbol “T” and risk phrase R60 (fertility) if they contain one or more substances producing such effects to which is assigned the risk phrase R60 which denotes substances toxic for reproduction of category 1 and category 2 in individual concentrations equal to or exceeding—
(a)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(b)the concentration specified in paragraph 6 of Part II of this Schedule (Table VI or Table VIA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits.
(2) Preparations shall be classified as toxic for reproduction category 3 and assigned the symbol “Xn” and the risk phrase R62 (fertility) if they contain one or more substances producing such effects to which is assigned the risk phrase R62 which denotes substances toxic for reproduction in category 3 in individual concentrations equal to or exceeding—
(a)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(b)the concentration specified in paragraph 6 of Part II of this Schedule (Table VI or Table VIA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits.
(3) Preparations shall be classified as toxic for reproduction category 1 or 2 and assigned the symbol “T” and risk phrase R61 (development) if they contain one or more substances producing such effects to which is assigned the risk phrase R61 which denotes substances toxic for reproduction of category 1 and category 2 in individual concentrations equal to or exceeding—
(a)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(b)the concentration specified in paragraph 6 of Part II of this Schedule (Table VI or Table VIA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits.
(4) Preparations shall be classified as toxic for reproduction category 3 and assigned the symbol “Xn” and the risk phrase R63 (development) if they contain one or more substances producing such effects to which is assigned the risk phrase R63 which denotes substances toxic for reproduction in category 3 in individual concentrations equal to or exceeding—
(a)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(b)the concentration specified in paragraph 6 of Part II of this Schedule (Table VI or Table VIA) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits.
Classification of preparations for environmental hazards
16.—(1) The environmental hazards of a preparation shall be assessed by one or more of the following methods—
(a)by the conventional method described in paragraphs 18 and 19 using concentration limits; or
(b)by the criteria referred to in the approved classification and labelling guide in relation to the preparation for an appropriate classification and label.
(2) Where the environmental hazards have been established by both methods, the results of the method referred to in sub-paragraph (1)(b) shall be used for classifying the preparation.
(3) Subject to sub-paragraph (4), for preparations of a known composition, with the exception of plant protection products, classified in accordance with the method set out in sub-paragraph (1)(b), a new assessment of the environmental hazards shall be performed either by the method set out in sub-paragraph (1)(a) or (1)(b) whenever—
(a)changes of composition of the initial concentration, as a weight/weight or volume/volume percentage, of one or more of the dangerous constituents are introduced by the manufacturer which exceed the permitted variations set out in the following table—
| Initial concentration range of the constituent | Permitted variation in actual concentration of the constituent | |
|---|---|---|
| ≤2.5% | ±30% | |
| >2.5 | ≤10% | ±20% |
| >10 | ≤25% | ±10% |
| >25 | ≤100% | ±5% |
(b)changes of composition involving the substitution or addition of one or more constituents, which may or may not be dangerous within the definitions in Schedule 1, are introduced by the manufacturer.
(4) The revised assessment required by paragraph (3) shall not be required where there is a valid scientific justification for considering that a re-evaluation of the hazard will not result in a change of classification.
Use of concentration limits in classification for environmental effects
17.—(1) In accordance with paragraph 16(1)(a), the environmental hazards shall be assessed by the conventional method described in paragraphs 18 and 19 using concentration limits.
(2) Where the substances concerned are dangerous substances and are listed as dangerous substances in Table 3.2 of part 3 of Annex VI of the CLP Regulation and are assigned concentration limits necessary for the application of the method of assessment described below, these concentration limits shall be used.
(3) Where the substances concerned are dangerous substances and do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation as dangerous substances or appear there without the concentration limits necessary for the application of the method of evaluation described below, the concentration limits shall be assigned in accordance with Part III of this Schedule.
Conventional method for the evaluation of hazards to the aquatic environment
18.—(1) The following preparations shall be classified as dangerous for the environment and assigned the symbol “N”, the indication of danger “dangerous for the environment” and the risk phrases R50 and R53 (R50-53)—
(a)preparations containing one or more substances classified as dangerous for the environment and to which is assigned risk phrases R50-53 in individual concentrations equal to or greater than—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in Part III of this Schedule (Table 1) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits;
(b)preparations containing more than one substance classified as dangerous to the environment and to which is assigned risk phrases R50-53 in lower individual concentrations than the limits specified under paragraph (a) if—
where—
Pn. R50-53 is the percentage by weight of each substance dangerous for the environment to which is assigned risk phrases R50-53 in the preparation,
Ln. R50-53 is the limit R50-53 for each substance dangerous for the environment to which is assigned the risk phrases R50-53 expressed as a percentage by weight.
(2) The following preparations shall be classified as dangerous for the environment and assigned the symbol “N”, the indication of danger “dangerous for the environment” and risk phrases R51 and R53 (R51-53) unless the preparation is already classified according to sub-paragraph (1)—
(a)preparations containing one or more substances classified as dangerous for the environment and to which is assigned risk phrases R50-53 or R51-53 in individual concentrations equal to or greater than—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in Part III of this Schedule (Table 1) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits;
(b)preparations containing more than one substance classified as dangerous to the environment and to which is assigned risk phrases R50-53 or R51-53 in lower individual concentrations than the limits specified under paragraph (a) if—
where—
Pn. R50-53 is the percentage by weight of each substance dangerous for the environment to which is assigned risk phrases R50-53 in the preparation,
Pn. R51-53 is the percentage by weight of each substance dangerous for the environment to which is assigned risk phrases R51-53 in the preparation,
Ln. R51-53 is the respective limit R51-53 for each substance dangerous for the environment to which is assigned the risk phrases R50-53 or R51-53 expressed as a percentage by weight.
(3) The following preparations shall be classified as dangerous for the environment and assigned the risk phrases R52 and R53 (R52-53) unless the preparation is already classified according to sub-paragraph (1) or (2)—
(a)preparations containing one or more substances classified as dangerous for the environment and to which is assigned risk phrases R50-53 or R51-53 or R52-53 in individual concentrations equal to or greater than—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in Part III of this Schedule (Table 1) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits;
(b)preparations containing more than one substance classified as dangerous for the environment and to which is assigned risk phrases R50-53 or R51-53 or R52-53 in lower individual concentrations than the limits specified under paragraph (a) if—R
where—
Pn. R50-sub5;3 is the percentage by weight of each substance dangerous for the environment to which is assigned risk phrases R50-53 in the preparation,
Pn. Rsub5;1-sub5;3 is the percentage by weight of each substance dangerous for the environment to which is assigned risk phrases R51-53 in the preparation,
PRsub5;2-sub5;3 is the percentage by weight of each substance dangerous for the environment to which is assigned risk phrases R52-53 in the preparation,
LRsub5;2-sub5;3 is the respective limit R52-53 for each substance dangerous for the environment to which is assigned the risk phrases R50-53, R51-53 or R52-53 expressed as a percentage by weight.
(4) The following preparations shall be classified as dangerous for the environment and assigned the symbol “N”, the indication of danger “dangerous for the environment” and the risk phrase R50 unless the preparation is already classified according to sub-paragraph (1)—
(a)preparations containing one or more substances classified as dangerous for the environment and to which is assigned risk phrase R50 individual concentrations equal to or greater than—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in Part III of this Schedule (Table 2) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits;
(b)preparations containing more than one substance classified as dangerous to the environment and to which is assigned risk phrase R50 in lower individual concentrations than the limits specified under paragraph (a) if—
where—
Pn. R50 is the percentage by weight of each substance dangerous for the environment to which is assigned risk phrase R50 in the preparation,
Ln. R50 is the limit R50 for each substance dangerous for the environment to which is assigned risk phrase R50 expressed as a percentage by weight.
(c)preparations containing one or more substances classified as dangerous to the environment and to which is assigned risk phrase R50 not meeting the criteria under paragraph (a) or (b) and containing one or more substances classified as dangerous to the environment and to which is assigned risk phrases R50-53 if—
where—
Pn. R50 is the percentage by weight of each substance dangerous for the environment to which is assigned risk phrase R50 in the preparation,
Pn. R50-53 is the percentage by weight of each substance dangerous for the environment to which is assigned risk phrases R50-53 in the preparation,
Ln. R50 is the respective limit R50 for each substance dangerous for the environment to which is assigned risk phrases R50 or R50-53 expressed as a percentage by weight.
(5) The following preparations shall be classified as dangerous for the environment and assigned the risk phrase R52 unless the preparation is already classified according to sub-paragraph (1), (2), (3) or (4)—
(a)preparations containing one or more substances classified as dangerous for the environment and to which is assigned risk phrase R52 in individual concentrations equal to or greater than—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in Part III of this Schedule (Table 3) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits;
(b)preparations containing more than one substance classified as dangerous to the environment and to which is assigned risk phrase R52 in lower individual concentrations than the limits specified under paragraph (a) if—
where—
P R52 is the percentage by weight of each substance dangerous for the environment to which is assigned risk phrase R52 in the preparation,
L R52 is the limit R52 for each substance dangerous for the environment to which is assigned risk phrase R52 expressed as a percentage by weight.
(6) The following preparations shall be classified as dangerous for the environment and assigned the risk phrase R53 unless the preparation is already classified according to sub-paragraph (1), (2) or (3)—
(a)preparations containing one or more substances classified as dangerous for the environment and assigned risk phrase R53 in individual concentrations equal to or greater than—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in Part III of this Schedule (Table 4) where the substance or substances do not appear in Annex Table 3.2 of part 3 of VI of the CLP Regulation or appear in it without concentration limits;
(b)preparations containing more than one substance classified as dangerous to the environment and to which is assigned risk phrase R53 in lower individual concentrations than the limits specified under paragraph (a) if—
where—
P R53 is the percentage by weight of each substance dangerous for the environment to which is assigned risk phrase R53 in the preparation,
L R53 is the limit R53 for each substance dangerous for the environment to which is assigned risk phrase R53 expressed as a percentage by weight.
(c)preparations containing one or more substances classified as dangerous to the environment and to which is assigned risk phrase R53 not meeting the criteria under paragraph (b) and containing one or more substances classified as dangerous to the environment and to which is assigned risk phrases R50-53, R51-53 or R52-53 if
where—
P53 is the percentage by weight of each substance dangerous for the environment to which is assigned risk phrase R53 in the preparation,
Pn. R50-53 is the percentage by weight of each substance dangerous for the environment to which is assigned risk phrases R50-53 in the preparation,
Pn. R51-53 is the percentage by weight of each substance dangerous for the environment to which is assigned risk phrases R51-53 in the preparation
PR52-53 is the percentage by weight of each substance dangerous for the environment to which is assigned risk phrases R52-53 in the preparation
LR53 is the respective limit R53 for each substance dangerous for the environment to which is assigned risk phrases R53, R50-53, R51-53 or R52-53 expressed as a percentage by weight.
Conventional method for the evaluation of hazards to the ozone layer
19. Preparations containing one or more substances classified as dangerous for the environment and to which is assigned the symbol “N” and the risk phrase R59 in individual concentrations equal to or greater than—
(i)either the concentration specified in Table 3.2 of part 3 of Annex VI of the CLP Regulation for the substance or substances under consideration, or
(ii)the concentration specified in Part III of this Schedule (Table 5) where the substance or substances do not appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation or appear in it without concentration limits,
shall be classified as dangerous for the environment and assigned the symbol “N”, the indication of danger “dangerous for the environment” and the risk phrase R59.
PART IICONCENTRATION LIMITS TO BE USED IN THE EVALUATION OF HEALTH HAZARDS
An assessment must be made of the health effects that the use of a substance or a preparation might entail. For that purpose the dangerous health effects have been subdivided into:
| 1. | acute lethal effects; |
| 2. | non-lethal irreversible effects after a single exposure; |
| 3. | severe effects after repeated or prolonged exposure; |
| 4. | corrosive effects, irritant effects; |
| 5. | sensitising effects; |
| 6. | carcinogenic effects, mutagenic effects, toxic effects for reproduction. |
The systematic assessment of the dangerous health effects is expressed by means of concentration limits, expressed as weight/weight percentage except for gaseous preparations (Tables A) where they are expressed as a volume/volume percentage and in conjunction with the classification of a substance.
The classification of the substance is expressed either by a symbol and one or more risk phrases or by categories (category 1, category 2 or category 3) also expressed by risk phrases when substances are shown to be carcinogenic, mutagenic or toxic for reproduction. Therefore it is important to consider, in addition to the symbol, all the phrases denoting specific risks which are assigned to each substance under consideration.
Acute lethal effects
Other than gaseous preparations
1.1 The concentration limits fixed in Table 1 determine the classification of the preparation in relation to the individual concentration of the substance(s) present whose classification is also shown.
Table I
| Classificationof the substance | Classification of the preparation | ||
|---|---|---|---|
| T+ | T | Xn | |
| T+ with R26, R27, R28 | concentration ≥ 7% | 1% concentration < 7% | 0.1% concentration < 1% |
| T with R23, R24, R25 | Concentration ≥ 25% | 3% ≤ concentration < 25% | |
| Xn with R20, R21, R22 | concentration ≥ 25% | ||
The R phrases denoting risk shall be assigned to the preparation in accordance with the following criteria—
(i)the label shall include one or more of the above mentioned R phrases according to the classification used,
(ii)in general, the R phrases selected should be those applicable to the substance(s) present in the concentration which gives rise to the most severe classification.
Gaseous preparations
1.2 The concentration limits expressed as a volume/volume percentage in Table IA determine the classification of the gaseous preparations in relation to the individual concentrations of the gas(es) present whose classification is also shown.
Table IA
| Classificationof the substance (gas) | Classification of the preparation | ||
|---|---|---|---|
| T+ | T | Xn | |
| T+ with R26, R27, R28 | concentration ≥ 1% | 0.2% ≤ concentration <1% | 0.02% ≤ concentration<0.2% |
| T with R23, R24, R25 | concentration ≥ 5% | 0.5% ≤ concentration <5% | |
| Xn with R20, R21, R22 | concentration ≥ 5% | ||
The R phrases denoting risk shall be assigned to the preparation in accordance with the following criteria—
(i)the label shall include one or more of the above mentioned R phrases according to the classification used,
(ii)in general, the R phrases selected should be those applicable to the substance(s) present in the concentration which gives rise to the most severe classification.
Non-lethal irreversible effects after a single exposure
Other than gaseous preparations
2.1 For substances that produce non-lethal irreversible effects after a single exposure (R39/route of exposure, R68/route of exposure), the individual concentration limits specified in Table II determine, when appropriate, the classification of the preparation.
Table II
| Classificationof the substance | Classification of the preparation | ||
|---|---|---|---|
| T+ | T | Xn | |
| T+ with R39/route of exposure | concentration ≥ 10% R39 (*) obligatory | 1% ≤ concentration <10% R39(*) obligatory | 0.1% ≤ concentration <1% R68(*) (†) obligatory |
| T with R39/ route of exposure | concentration ≥ 10% R39(*) obligatory | 1% ≤ concentration <10% R68(*) (†) obligatory | |
| Xn with R68/route of exposure | concentration ≥ 10% R68(*)(†) obligatory | ||
(*) In order to indicate the route of administration/ exposure the combined R phrases listed in Annex III of Council Directive 67/548/EEC shall be used.
(†) R68 here refers to substances classified as harmful. Concentration limits for substances required to be labelled R68 but classified as mutagenic are given in Table VI.
Gaseous preparations
2.2 For gases that produce non-lethal irreversible effects after a single exposure (R39/route of exposure, R68/route of exposure), the individual concentration limits specified in Table IIA, expressed as a volume/volume percentage, determine, when appropriate, the classification of the preparation.
Table IIA
| Classificationof the substance (gas) | Classification of the preparation | ||
|---|---|---|---|
| T+ | T | Xn | |
| T+ withR39/route ofexposure | concentration ≥ 1% R39(*) obligatory | 0.2% ≤ concentration <1% R39(*) obligatory | 0.02% ≤ concentration<0.2% R68(*)(†) obligatory |
| T with R39/route of exposure | concentration ≥ 5% R39(*) obligatory | 0.5% ≤ concentration <5% R68(*)(†) obligatory | |
| Xn with R68/route ofexposure | concentration ≥ 5% R68 (*)(†) obligatory | ||
(*) In order to indicate the route of administration/exposure the combined R phrases listed in Annex III of Council Directive 67/548/EEC shall be used.
(†) R68 here refers to substances classified as harmful. Concentration limits for substances required to be labelled R68 but classified as mutagenic are given in Table VI.
Severe effects after repeated or prolonged exposure
Other than gaseous preparations
3.1 For substances that produce severe effects after repeated exposure (R48/route of exposure), the individual concentration limits specified in Table III determine, when appropriate, the classification of the preparation.
Table III
| Classificationof the substance | Classification of the preparation | |
|---|---|---|
| T+ | Xn | |
| T with R48/ route of exposure | concentration ≥ 10% R48(*) obligatory | 1% ≤ concentration <10% R48(*) obligatory |
| Xn with R48/route of exposure | concentration ≥ 10% R48(*) obligatory | |
(*) In order to indicate the route of administration/exposure the combined R phraseslisted in Annex III of Council Directive 67/548/EEC shall be used.
Gaseous preparations
3.2 For gases that produce severe effects after repeated or prolonged exposure (R48/route of exposure), the individual concentration limits specified in Table IIIA, expressed as a volume/volume percentage, determine, when appropriate, the classification of the preparation.
Table IIIA
| Classificationof the substance (gas) | Classification of the preparation | |
|---|---|---|
| T+ | Xn | |
| T with R48/route of exposure | concentration ≥ 5% R48(*) obligatory | 0.5% ≤ concentration <5% R48(*) obligatory |
| Xn with R48/route of exposure | concentration ≥ 5% R48(*) obligatory | |
(*) In order to indicate the route of administration/exposure the combined R phraseslisted in Annex III of Council Directive 67/548/EEC shall be used.
Corrosive and irritant effects including serious damage to eye
Other than gaseous preparations
4.1 For substances that produce corrosive effects (R34, R35) or irritant effects (R36, R37, R38, R41), the individual concentration limits specified in Table IV determine, when appropriate, the classification of the preparation.
Table IV
| Classificationof the substance | Classification of the preparation | |||
|---|---|---|---|---|
| C with R35 | C with R34 | Xi with R41 | Xi withR36, R37, R38 | |
| C with R35 | concentration ≥ 10% R35 obligatory | 5% ≤ concentration <10% R34 obligatory | 5%(*) | 1% ≤ concentration <5% R36/38 obligatory |
| C with R34 | Concentration≥ 10% R34 obligatory | 10%(*) | 5% ≤ concentration <10% R36/38 obligatory | |
| Xi with R41 | concentration ≥ 10% R41 obligatory | 5% ≤ concentration<10% R36 obligatory | ||
| Xi withR36, R37, R38 | concentration ≥ 20%R36, R37, R38 are obligatory in the light of the concentration present if they apply to the substances under consideration | |||
(*) According to the approved classification and labelling guide, when a substance or preparation is classified as corrosive and assigned the risk phrase R34 or R35, the risk of severe damage to the eyes is considered implicit and the risk phrase R41 is not included on the label. Consequently, if the preparation contains corrosive substances with R35 or R34 below the concentration limits for a classification of the preparation as corrosive, such substances can contribute to a classification of the preparation as irritant (R41) or irritant (R36).
Note
Simple application of the conventional method to preparations containing substances classified as corrosive or irritant may result in under-classification or over-classification of the hazard, if other relevant factors (eg pH of the preparation) are not taken into account. Therefore, in classifying for corrosivity consider the advice given in the approved classification and labelling guide regarding classification as corrosive and paragraph 4(4)(b) and (c) of Part I of this Schedule.
Gaseous preparations
4.2 For gases that produce such effects (R34, R35 or R36, R37, R38, R41), the individual concentration limits specified in Table IVA, expressed as a volume/volume percentage determine, when appropriate the classification of the preparation.
Table IVA
| Classificationof the substance (gas) | Classification of the preparation | |||
|---|---|---|---|---|
| C with R35 | C with R34 | Xi with R41 | Xi withR36, R37, R38 | |
| C with R35 | Concentration≥ 1% R35 obligatory | 0.2% ≤ concentration <1% R34 obligatory | 0.2%(*) | 0.02% ≤ concentration < 0.2% R36/37/38 obligatory |
| C with R34 | Concentration ≥ 5% R34 obligatory | 5%(*) | 0.5% ≤ concentration < 5% R36/37/38 obligatory | |
| Xi with R41 | concentration ≥ 5% R41 obligatory | 0.5% ≤ concentration <5% R36 obligatory | ||
| Xi withR36, R37, R38 | concentration ≥ 5% R36, R37, R38 obligatory as appropriate | |||
(*) According to the approved classification and labelling guide, when a substance or preparation is classified as corrosive and assigned the risk phrase R34 or R35, the risk of severe damage to the eyes is considered implicit and the risk phrase R41 is not included on the label. Consequently, if the preparation contains corrosive substances with R35 or R34 below the concentration limits for a classification of the preparation as corrosive, such substances can contribute to a classification of the preparation as irritant (R41) or irritant (R36).
Note
Simple application of the conventional method to preparations containing substances classified as corrosive or irritant may result in under-classification or over-classification of the hazard, if other relevant factors (eg pH of the preparation) are not taken into account. Therefore, in classifying for corrosivity, consider the advice given in the approved classification and labelling guide regarding classification as corrosive and paragraph 4(4)(b) and (c) of Part I of this Schedule.
Sensitising effects
Other than gaseous preparations
5.1 Preparations that produce such effects are classified as sensitising and assigned:
the symbol Xn and phrase R42 if this effect can be produced by inhalation,
the symbol Xi and phrase R43 if this effect can be produced through contact with the skin.
The individual concentration limits specified in Table V determine, when appropriate, the classification of the preparation.
Table V
| Classification of the substance | Classification of the preparation | |
|---|---|---|
| Sensitising with R42 | Sensitising with R43 | |
| Sensitising with R42 | concentration ≥ 1% R42 obligatory | |
| Sensitising with R43 | concentration ≥ 1% R43 obligatory | |
Gaseous preparations
5.2 Gases that produce such effects are classified as sensitising and assigned:
the symbol Xn and phrase R42 if this effect can be produced by inhalation,
the symbol Xi and phrase R43 if this effect can be produced by inhalation and through contact with the skin.
The individual concentration limits specified in Table VA expressed as a volume/volume percentage, determine, when appropriate, the classification of the preparation.
Table VA
| Classification of the substance (gas) | Classification of the preparation | |
|---|---|---|
| Sensitising with R42 | Sensitising with R43 | |
| Sensitising with R42 | concentration ≥ 0.2% R42 obligatory | |
| Sensitising with R43 | concentration ≥ 0.2% R43 obligatory | |
Carcinogenic/mutagenic/toxic effects for reproduction
Other than gaseous preparations
6.1 For substances which produce such effects and for which specific concentration limits do not yet appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation, concentration limits laid down in Table VI shall determine, where appropriate, the classification of the preparation.
The following symbol and risk phrases are assigned:
Carcinogenic categories 1 and 2: T; R45 or R49 Carcinogenic category 3: Xn; R40 Mutagenic categories 1 and 2: T; R46 Mutagenic category 3: Xn R68 Toxic for reproduction fertility categories 1 and 2: T; R60 Toxic for reproduction development categories 1 and 2: T; R61 Toxic for reproduction fertility category 3: Xn; R62 Toxic for reproduction development category 3: Xn; R63 Table VI
Classification of the substance Classification of the preparation Categories 1 and 2 Category 3 Carcinogenic substancesof category 1 or 2 with R45 or R49 concentration ≥ 0.1%carcinogenic
R45, R49 obligatory as appropriate
Carcinogenic substancesof category 3 with R40 concentration ≥ 1%carcinogenic
R40 obligatory (unless already assigned R45(*))
Mutagenic substances of category 1 or 2 with R46 concentration ≥ 0.1% mutagenic
R46 obligatory
Mutagenic substances of category 3 with R68(**) concentration ≥ 1%mutagenic
R68(**) obligatory (unless already assigned R46)
Substances “toxic for reproduction” of category 1 or 2 with R60 (fertility) concentration ≥ 0.5%toxic for reproduction(fertility)
R60 obligatory
Substances “toxic for reproduction” of category 3 with R62 (fertility) concentration ≥ 5%toxic for reproduction(fertility)
R62 obligatory (unless already assigned R60)
Substances “toxic for reproduction” of category1 or 2 with R61 (development) concentration ≥ 0.5%toxic for reproduction (development)
R61 obligatory
Substances “toxic for reproduction” of category 3 with R63 (development) concentration ≥ 5%toxic for reproduction (development)
R63 obligatory (unless already assigned R61)
(*) In cases where the preparation is assigned R49 and R40, both R phrases shall be kept, because R40 does not distinguish between the exposure routes, whereas R49 is only assigned for the inhalation route.
(**) R68 here refers to substances classified as mutagenic. Concentration limits for substances required to be labelled R68 but classified as harmful are given in Table II.
Gaseous preparations
6.2 For gases which produce such effects and for which specific concentration limits do not yet appear in Table 3.2 of part 3 of Annex VI of the CLP Regulation, concentration limits laid down in Table VIA, expressed as a volume/volume percentage, shall determine, where appropriate, the classification of the preparation.
The following symbol and risk phrases are assigned:
Carcinogenic categories 1 and 2: T; R45 or R49 Carcinogenic category 3: Xn; R40 Mutagenic categories 1 and 2: T; R46 Mutagenic category 3: Xn; R68 Toxic for reproduction fertility categories 1 and 2: T; R60 Toxic for reproduction development categories 1 and 2: T; R61 Toxic for reproduction fertility category 3: Xn; R62 Toxic for reproduction development category 3: Xn; R63 Table VIA
Classification of the substance (gas) Classification of the preparation Categories 1 and 2 Category 3 Carcinogenic substances ofcategory 1 or 2 with R45 or R49 concentration ≥ 0.1%carcinogenic R45, R49 obligatory asappropriate Carcinogenic substances ofcategory 3 with R40 concentration ≥ 1%carcinogenic R40
obligatory (unless already assigned R45(*))
Mutagenic substances ofcategory 1 or 2 with R46 concentration ≥ 0.1% mutagenic
R46 obligatory
Mutagenic substances ofcategory 3 with R68(**) concentration ≥ 1%mutagenic
R68(**) obligatory (unless already assigned R46)
Substances “toxic forreproduction” of category 1 or 2
with R60 (fertility)
concentration ≥ 0.2%toxic for reproduction (fertility)
R60 obligatory
Substances “toxic forreproduction” of category 3
with R62 (fertility)
concentration ≥ 1%toxic for reproduction(fertility)
R62 obligatory (unless already assigned R60)
Substances “toxic forreproduction” of category 1 or 2
with R61 (development)
concentration ≥ 0.2%toxic for reproduction(development)
R61 obligatory
Substances “toxic forreproduction” of category 3
with R63 (development)
concentration ≥ 1% toxic for reproduction (development)
R63 obligatory (unless already assigned R61)
(*) In cases where the preparation is assigned R49 and R40, both R phrases shall be kept, because R40 does not distinguish between the exposure routes, whereas R49 is only assigned for the inhalation route.
(**) R68 here refers to substances classified as mutagenic. Concentration limits for substances required to be labelled R68 but classified as harmful are given in Table IIA.
PART IIIConcentration limits to be used for the evaluation of Environment Hazards
The aquatic environment
1. The concentration limits fixed in the following tables, expressed as a weight/weight percentage, determine the classification of the preparation in relation to the individual concentration of the substances present whose classification is also shown.
Table 1a
Acute aquatic toxicity and long-term adverse effects
Classification of the substance Classification of the preparation N, R50-53
N, R51-53
R52-53
N,R50-53
see Table 1b
N, R51-53
See Table 1b
Cn ≥25%
R52-53
see Table 1b
2.5%≤ Cn <25%
Cn≥25%
For preparations containing a substance classified with N, R50-53, the concentration limits and the resulting classification given in Table 1b are applicable.
Table 1b
Acute acquatic toxicity and long-term adverse effects of substance very toxic to the aquatic environment
LG50 or EC50 value (“L(E)C50”) of substance classified as N, R50-53 (mg/l) Classification of the preparation N, R50-53 N, R51-53 R52-53 0.1 <L(E)C50 ≤1 Cn ≥ 25% 2.5% ≤ Cn <25% 0.25% ≤ Cn <2.5% 0.01 <L(E)C50 ≤0.1 Cn ≥ 2.5% 0.25% ≤ Cn <2.5% 0.025% ≤ C*subn; <0.25% 0.001 <L(E)C50 ≤0.01 Cn ≥ 0.25% 0.025% ≤ Cn <0.25% 0.0025% ≤ Cn <0.025% 0.0001 <L(E)C50 ≤0.001 Cn ≥ 0.025% 0.0025% ≤ Cn <0.025% 0.00025% ≤ Cn <0.0025% 0.00001 <L(E)C50 ≤0.0001 Cn ≥ 0.0025% 0.00025% ≤ Cn <0.0025% 0.000025% ≤ Cn <0.00025% For preparations containing substances with a lower LC50 or EC50 value than 0.00001 mg/l, the corresponding concentration limits are calculated accordingly (in factor 10 intervals).
Table 2
Acute aquatic toxicity
LC50 or EC50 value (“L(E)C50”) of substance classified either as N, R50 or as N, R50-53 (mg/l) Classification of the preparation N, R50 0.1 L(E)C50 ≤1 Cn≥ 25% 0.01 L(E)C50 ≤0.1 Cn≥ 2.5% 0.001 L(E)C50 ≤0.01 Cn≥ 0.25% 0.0001 L(E)C50 ≤0.001 Cn≥ 0.025% 0.00001 L(E)C50 ≤0.0001 Cn≥ 0.0025% For preparations containing substances with a lower LC50 or EC50 value than 0.00001 mg/l, the corresponding concentration limits are calculated accordingly (in factor 10 intervals).
Table 3
Aquatic toxicity
Classification of the substance Classification of the preparation R52 R52 Cn ≥25% Table 4
Long-term adverse effects
Classification of the substance Classification of the preparation R53 R53 Cn ≥25% N, R50-53 Cn ≥25% N, R51-53 Cn ≥25% R52-53 Cn ≥25%
The non-aquatic environment
2. The concentration limits fixed in the following table, expressed as a weight/weight percentage or, for gaseous preparations as a volume/volume percentage, determine the classification of the preparation in relation to the individual concentration of the substances present whose classification is also shown.
Table 5
Dangerous for the ozone layer
| Classification of the substance | Classification of preparation N, R59 |
|---|---|
| N with R59 | Cn ≥ 0.1% |
Regulations 7 and 9
SCHEDULE 4LABELLING PARTICULARS FOR DANGEROUS SUBSTANCES, DANGEROUS PREPARATIONS AND FOR CERTAIN OTHER PREPARATIONS
PART 1GENERAL PROVISIONS RELATING TO LABELS
Labelling particulars for dangerous substances
1.—(1) In the case of a dangerous substance which is listed in Table 3.2 of part 3 of Annex VI of the CLP Regulation, the particulars to be shown on the label in accordance with regulation 7(2)(c) shall be the particulars specified for that dangerous substance in the relevant entry in that table.
(2) Subject to paragraph 4, in the case of a dangerous substance which is not listed in Table 3.2 of part 3 Annex VI of the CLP Regulation, the particulars required to be shown on the label in accordance with regulation 7(2)(c) shall be determined from the classification of the substance in accordance with regulation 4 in conjunction with the approved classification and labelling guide.
Labelling particulars for dangerous preparations
2.—(1) Subject to paragraphs 3 and 4, the provisions of this paragraph shall have effect in relation to the labelling of dangerous preparations.
(2) Subject to sub-paragraph (3), the particulars relating to the chemical name required to be shown on the label in accordance with regulation 7(3)(c)(i) shall be shown according to the following rules—
(a)in the case of a dangerous preparation classified as requiring the indication of danger T+, T or Xn, only substances requiring those indications of danger present in the dangerous preparation in concentrations equal to or greater than—
(i)the lowest limit (the Xn limit) for the substance laid down in Table 3.2 of part 3 of Annex VI of the CLP Regulation, or
(ii)where there is no such limit, the relevant limit laid down in Part II of Schedule 3
,have to be taken into consideration;
(b)in the case of a dangerous preparation classified as requiring the indication of danger C, only substances requiring that indication of danger present in the dangerous preparation in concentrations equal to or greater than—
(i)the lowest limit (the Xi limit) for the substance laid down in Table 3.2 of part 3 of Annex VI of the CLP Regulation, or
(ii)where there is no such limit, the relevant limit laid down in Part II of Schedule 3,
have to be taken into consideration;
(c)if the dangerous preparation is assigned one or more of the following danger categories:
carcinogen category 1, 2 or 3,
mutagen category 1, 2 or 3,
toxic for reproduction category 1, 2 or 3,
very toxic, toxic or harmful due to non-lethal effects after a single exposure,
toxic or harmful due to severe effects after repeated or prolonged exposure,
sensitising,
the name of any substance causing the dangerous preparation to be so assigned shall be referred to;
(d)as a consequence of the provisions set out in paragraphs (a) to (c), the name of any substance which led to the classification of the dangerous preparation in the following danger categories:
explosive,
oxidising,
extremely flammable,
highly flammable,
flammable,
irritant,
dangerous for the environment,
need not be referred to on the label unless so required by paragraph (a), (b) or (c).
(3) The chemical name referred to in sub-paragraph (2) shall be—
(a)in the case of a substance listed in Table 3.2 of part 3 of Annex VI of the CLP Regulation, the name or one of the names under which that substance is listed; or
(b)in the case of a substance not so listed, an internationally recognised name.
(4) For the purpose of labelling, no account shall be taken of a substance in the dangerous preparation where the concentration of that substance is less than the concentration referred to in paragraph 6 of Part 1 of Schedule 3.
(5) Subject to sub-paragraph (4), the particulars to be shown on the label in accordance with regulation 7(3)(c)(ii), (iii) and (iv) shall be determined from the classification of the dangerous preparation made in accordance with regulation 4 in conjunction with the approved classification and labelling guide.
(6) As a general rule, a maximum of four chemical names shall suffice to identify the substances primarily responsible for the major health hazards which have given rise to the classification and the choice of the corresponding risk phrases—although in some cases more than four chemical names may be necessary.
Confidentiality of chemical names
3.—(1) Subject to sub-paragraph (2), where the supplier of a dangerous preparation is able to demonstrate to the Executive that the disclosure on the label or safety data sheet of the chemical identity of a substance which is exclusively classified as—
(a)irritant with the exception of those assigned R41 or irritant in combination with one or more of the other properties mentioned in paragraph (2)(2)(d); or
(b)harmful or harmful in combination with one or more of the properties mentioned in paragraph (2)(2)(d) presenting acute lethal effects alone,
will put at risk the confidential nature of the supplier’s intellectual property, that supplier shall, in accordance with the provisions of Annex VI of Council Directive 1999/45/EC, be permitted to refer to that substance either by means of a name that identifies the most important functional chemical groups or by means of an alternative name.
(2) The derogation in sub-paragraph (1) shall not apply in respect of a substance which has been assigned a Community exposure limit.
(3) Where a supplier wishes to take advantage of the derogation contained in sub-paragraph (1), the supplier shall make application to the Executive accordingly, enclosing the information specified in Annex VI to Council Directive 1999/45/EC.
(4) The Executive may require such further information from the supplier as is necessary to determine the validity of an application made under sub-paragraph (3).
Indications of danger and symbols for dangerous substances and dangerous preparations
4.—(1) Except in the case of a dangerous substance which is listed in Table 3.2 of part 3 of Annex VI of the CLP Regulation, where a dangerous substance or dangerous preparation is required to have more than one indication of danger in either of the following groups listed in decreasing order of severity, namely—
(a)explosive, oxidising, extremely flammable and highly flammable; or
(b)very toxic, toxic, corrosive, harmful and irritant,
only one of the indications of danger with its symbol from each group corresponding to the most severe indication of danger in that group need be shown.
(2) The risk phrases R12 (extremely flammable) and R11 (highly flammable) need not be used if they repeat the indication of danger shown on the label.
PART IIPARTICULAR PROVISIONS CONCERNING CERTAIN PREPARATIONS
ASPECIAL PROVISIONS APPLYING TO DANGEROUS PREPARATIONS
Dangerous preparations to be supplied to the general public
1.—(1) The label on the packaging of dangerous preparations intended to be supplied to the general public must in addition to the relevant safety advice bear the relevant safety phrase S1, S2, S45 or S46 in accordance with the approved classification and labelling guide.
(2) When the dangerous preparations referred to in sub-paragraph (1) are classified as very toxic, toxic or corrosive and where it is physically impossible to give the information on the package itself, packages containing such preparations must be accompanied by precise and easily understandable instructions for use including, where appropriate, instructions for the destruction of the empty package.
Dangerous preparations intended for use by spraying
2. The label on the packaging containing dangerous preparations intended to be used for spraying shall bear the safety phrase S23 and safety phrase S38 or S51 assigned in accordance with the approved classification and labelling guide.
Dangerous preparations containing a substance affected by the risk phrase R33 (danger of cumulative effects)
3. When a dangerous preparation contains at least one substance required to show the risk phrase R33, that phrase must be shown on the label on the packaging of the dangerous preparation when the concentration of that substance is equal to or higher than 1% unless a different value is shown for that substance in Table 3.2 of part 3 of Annex VI of the CLP Regulation.
Dangerous preparations containing a substance affected by the risk phrase R64 (may cause harm to breast-fed babies)
4. When a dangerous preparation contains at least one substance required to show the risk phrase R64, that phrase must be shown on the label on the packaging of the dangerous preparation when the concentration of that substance is equal to or higher than 1% unless a different value is shown for that substance in Table 3.2 of part 3 of Annex VI of the CLP Regulation.
BSPECIAL PROVISIONS APPLYING TO ANY PREPARATION
Paints and varnishes containing lead
1.—(1) The label on the packaging of paints and varnishes containing lead in quantities exceeding 0.15% (expressed as weight of lead out of the total weight of the preparation and determined in accordance with ISO Standard 6503/1984) shall bear the following inscription—
“Contains lead. Should not be used on surfaces that are liable to be chewed or sucked by children.”.
(2) In the case of packages containing less than 125 millilitres of the preparations referred to in sub-paragraph (1), the inscription on the label may be—
“Warning! Contains lead.”.
Cyanoacrylate based adhesives
2.—(1) The label on the immediate packaging of glues based on cyanoacrylates shall bear the following inscription—
“Cyanoacrylate.
Danger.
Bonds skin and eyes in seconds.
Keep out of the reach of children.”.
(2) Appropriate safety advice shall accompany the package.
Preparations containing isocyanates
3.—(1) The label on the packaging of preparations containing isocyanates (whether as monomers, oligomers, prepolymers etc. or as preparations thereof) shall bear the following inscriptions—
“Contains isocyanates.
See information supplied by the manufacturer.”.
Certain preparations containing epoxy constituents
4. The label on the packaging of preparations containing epoxy constituents with an average molecular weight ≤ 700 shall bear the following inscription—
“Contains epoxy constituents.
See information supplied by the manufacturer.”.
Preparations intended to be sold to the general public that contain active chlorine
5. The label on the packaging of preparations containing more than 1% of active chlorine which are intended to be sold to the general public shall bear the following inscription—
“Warning! Do not use together with other products. May release dangerous gases (chlorine).”.
Preparations containing cadmium (alloys) intended to be used for brazing or soldering
6. The label on the packaging of preparations containing cadmium (alloys) intended to be used for brazing or soldering shall bear the following inscriptions—
“Warning! Contains cadmium.
Dangerous fumes are formed during use.
See information supplied by the manufacturer.
Comply with the safety instructions.”.
Preparations not classified as sensitising but containing at least one sensitising substance
7. The label on the packaging of preparations containing at least one substance classified as sensitising and being present in a concentration ≥ 0.1% or in a concentration greater than or equal to that specified under a specific note for the substance in Table 3.2 of part 3 of Annex VI of the CLP Regulation must bear the inscription—
“Contains (name of sensitising substance). May produce an allergic reaction.”.
Liquid preparations containing halogenated hydrocarbons
8. For liquid preparations which show no flashpoint or a flashpoint higher than 55°C and contain a halogenated hydrocarbon and more than 5% flammable or highly flammable substances, the label on the packaging must bear the following inscription as appropriate—
“Can become highly flammable in use. Or
Can become flammable in use.”.
Preparations containing a substance assigned the risk phrase R67
9. When a preparation contains one or more substances assigned the risk phrase R67, the label on the packaging of the preparation must bear the following inscription—
“Vapours may cause drowsiness and dizziness,
when the total concentration of such substances present in the preparation is ≥15%, unless:
the preparation is already classified with phrases R20, R23, R26, R68/20, R39/23 or R39/26, or
the preparation is in a package not exceeding 125 ml.”
Cement and cement preparations
10.—(1) The label on the packaging of any cement or cement preparation which would contain, when hydrated, more than 0.0002% soluble chromium (VI) of the total dry weight of the cement but for the use of reducing agents shall be marked with information on the packing date, and on the storage conditions and the storage period appropriate to maintaining the activity of the reducing agent and to preventing the content of soluble chromium (VI) from exceeding 0.0002% of the total dry weight of the cement, unless it is supplied or used for controlled, closed and totally automated processes in which cement and cement-containing preparations are handled solely by machines and in which there is no possibility of contact with the skin.
(2) The label on the packaging of any cement or cement preparation containing more than 0.0002% soluble chromium (VI) of the total dry weight of the cement must bear the inscription—
“Contains chromium (VI). May produce an allergic reaction.”
unless the preparation is already classified and labelled as a sensitiser with risk phrase R43.
CSPECIAL PROVISIONS APPLYING TO CERTAIN OTHER PREPARATIONS
Preparations not intended for the general public
11. The label on the packaging of a preparation of the type specified in Article 31(3) of REACH must bear the following inscription—
“Safety data sheet available for professional user on request.”.
Regulation 11
SCHEDULE 5BRITISH AND INTERNATIONAL STANDARDS RELATING TO CHILD RESISTANT FASTENINGS AND TACTILE WARNING DEVICES
The British Standards and International Standards referred to in regulation 11 are as follows—
“BS EN 28317” means the British Standard Specification for packagings resistant to opening by children, BS EN 28317: 1993 which was published by the British Standards Institution and came into effect on 15 February 1993;
“ISO 8317” means the International Standard ISO 8317 (1 July 1989 edition) relating to “Child-resistant packagings—Requirements for the testing of reclosable packages” adopted by the International Standards Organisation;
“BS 6652” means the British Standard Specification for packagings resistant to opening by children, BS 6652: 1989 which was published by the British Standards Institution and came into effect on 30 June 1989;
“EN 862” means the CEN standard EN 862 (March 1997 edition) relating to “Packaging—Child-resistant packaging—Requirements and testing procedures for non-reclosable packages for non-pharmaceutical products” adopted by the European Committee for Standardisation;
“EN ISO 11683” means the EN ISO Standard 11683 (1997 Edition) relating to “Packaging—Tactile warnings of danger—Requirements.”;
“BS 7501” means the British Standard on the general criteria for the operation of testing laboratories BS 7501: 1989 which was published by the British Standards Institution and came into effect on 31 October 1989;
“EN 45 000” means the European Standards Series 45 000 which sets out the general criteria which laboratories must adhere to in order to obtain accreditation for the certification of child resistant fastenings.
Regulation 17
SCHEDULE 6AMENDMENTS
| Regulations to be amended | Regulations and Schedules to be amended | Amendments to be made |
|---|---|---|
| The Pipelines Safety Regulations 1996(46) | Paragraph 10 of Schedule 2 | For “regulation 5 of the Chemicals (Hazard Information and Packaging for Supply) Regulations 1994;” substitute “regulation 4 of the Chemicals (Hazard Information and Packaging for Supply) Regulations 2009;”. |
| The Health and Safety (Enforcing Authority) Regulations 1998(47) | Regulation 2(1) | After the definition of ““construction work” and “contractor”” insert—
|
| Paragraph 1(b) of Schedule 1 | For “substance or preparation dangerous for supply” substitute “dangerous substance or dangerous preparation”. | |
| The Biocidal Products Regulations 2001(48) | Regulation 2(1) | For the definition of “the 2002 Regulations” substitute ““the 2009 Regulations” means the Chemicals (Hazard Information and Packaging for Supply) Regulations 2009;”. Omit the definition of “approved supply list”. In the definition of “classified” for “the 2002 Regulations” substitute “the 2009 Regulations”. After the definition of “classified” add the definition ““The CLP Regulation” means Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006;” |
| Regulation 2(7)(a) | For “Part I of the approved supply list” substitute “Table 3.2 of part 3 of Annex VI of the CLP Regulation;”. | |
| Regulation 2(7)(b) | For “Part I of the approved supply list” substitute “Table 3.2 of part 3 of Annex VI of the CLP Regulation;”. | |
| Regulation 2(7)(c)(i) | For “Part I of the approved supply list” substitute “Table 3.2 of part 3 of Annex VI of the CLP Regulation;”. | |
| Regulation 2(7)(d)(i) | For “Part I of the approved supply list” substitute “Table 3.2 of part 3 of Annex VI of the CLP Regulation;”. | |
| Paragraph 13 of Schedule 4 | For “the 2002 Regulations,” substitute “the 2009 Regulations,” and for “regulation 6 of those Regulations.” substitute “Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals.” | |
| Paragraph 6(a) of Schedule 6 | For “Part I of the approved supply list” substitute “Table 3.2 of part 3 of Annex VI of the CLP Regulation;”. | |
| Paragraph 6(b) of Schedule 6 | For “the 2002 Regulations” substitute “the 2009 Regulations;”. | |
| Paragraph 4 of Schedule 7 | For “the 2002 Regulations” substitute “the 2009 Regulations”. | |
| The Control of Lead at Work Regulations 2002(49) | Regulation 2(1) | In the definition of “safety data sheet” for “the Chemicals (Hazard Information and Packaging for Supply) Regulations 2002;” substitute “the Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals.” |
| The Control of Substances Hazardous to Health Regulations 2002(50) | Regulation 2(1) | In the definition of “the CHIP Regulations” for “the Chemicals (Hazard Information and Packaging for Supply) Regulations 2002;” substitute “the Chemicals (Hazard Information and Packaging for Supply) Regulations 2009;”. |
| Omit the definition of “the approved supply list”. | ||
| After the definition of “the CHIP Regulations” add the definition ““The CLP Regulation” means Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006;”. | ||
| In the definition of “safety data sheet” for “regulation 5 of the CHIP Regulations;” substitute “Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals.” | ||
| In the definition of “substances hazardous to health” for “Part I of the approved supply list as dangerous for supply within the meaning of the CHIP Regulations” substitute “Table 3.2 of part 3 of Annex VI of the CLP Regulation;”. | ||
| The Dangerous Substances and Explosive Atmospheres Regulations 2002(51) | Regulation 2 | In the definition of “the CHIP Regulations” for “the Chemicals (Hazard Information and Packaging for Supply) Regulations 2002;” substitute “the Chemicals (Hazard Information and Packaging for Supply) Regulations 2009;”. |
| In the definition of “safety data sheet” for “regulation 5 of the CHIP Regulations;” substitute “Regulation (EC) 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals.” | ||
| The Control of Asbestos Regulations 2006(52) | Schedule 2, paragraph 1(1)(a) | For “the Chemicals (Hazard Information and Packaging for Supply) Regulations 2002” substitute “the Chemicals (Hazard Information and Packaging for Supply) Regulations 2009”. |
| The REACH Enforcement Regulations 2008(53) | Part 1 of Schedule 3, paragraph 1, sub-paragraph (p)(i) | For “the Chemicals (Hazard Information and Packaging for Supply) Regulations 2002;” substitute “the Chemicals (Hazard Information and Packaging for Supply) Regulations 2009;”. |
Regulation 18
SCHEDULE 7REVOCATIONS
| Regulations revoked | References | Extent of Revocation |
|---|---|---|
| The Chemicals (Hazard Information and Packaging for Supply) Regulations 2002 | S.I. 2002/1689 | The whole Regulations. |
| The Control of Substances Hazardous to Health (Amendment) Regulations 2004 | S.I. 2004/3386 | Regulation 3. |
| The Chemicals (Hazard Information and Packaging for Supply) (Amendment) Regulations 2005 | S.I. 2005/2571 | The whole Regulations. |
| The Legislative Reform (Health and Safety Executive) Order 2008 | S.I. 2008/960 | The reference to the Chemicals (Hazard Information and Packaging for Supply) Regulations 2002 in Schedule 3. |
| The Export and Import of Dangerous Chemicals Regulations 2008 | S.I. 2008/2108 | Regulation 5 paragraphs (2) and (4). |
| The Chemicals (Hazard Information and Packaging for Supply) (Amendment) Regulations 2008 | S.I. 2008/2337 | The whole Regulations. |
| The REACH Enforcement Regulations 2008 | S.I. 2008/2852 | Paragraphs 1 to 4 of Part 3 of Schedule 10. |
Explanatory Note
(This note is not part of the Order)
1. These Regulations consolidate, revoke and re-enact with amendments the Chemicals (Hazard Information and Packaging for Supply) Regulations 2002.
2. These Regulations, as respects Great Britain—
(a)provide for the enforcement of Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures (OJ No L353, 31.12.08, p. 1) (“the CLP Regulation”), in addition to the enforcement of these Regulations;
(b)implement parts of Directive 2006/121/EC (OJ No L396, 30.12.06, p.850) of the European Parliament and the Council of 18 December 2006 on the approximation of laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances in order to adapt it to Regulation (EC) No 1907/2006 (OJ L142, 31.5.08, p. 1) concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (“REACH”) and establishing a European Chemicals Agency;
(c)implement Council Directive 1992/32/EEC (OJ No. L154, 5.6.92, p. 1) amending for the 7th time Council Directive 67/548/EEC (OJ No. L196, 16.8.67, p. 1), in so far as its provisions relate to the classification, packaging and labelling of dangerous substances (“the substances Directive”); and
(d)implement Council Directive 1999/45/EC (OJ No. L200, 30.7.99, p. 1) on the classification, packaging and labelling of dangerous preparations (“the preparations Directive”).
3. These Regulations also implement the Directives referred to below which adapt to technical progress and modify the substances Directive and the preparations Directive. These Directives are—
(a)Commission Directive 91/410/EEC (OJ No L228, 17.8.91, p. 67) 14th adaptation to technical progress of the substances Directive;
(b)Commission Directive 93/21/EEC (OJ No L110, 4.5.93, p. 20), 18th adaptation to technical progress of the substances Directive;
(c)Commission Directive 2000/32/EC (OJ No L136, 8.6.2000, p. 1) 26th adaptation to technical progress of the substances Directive;
(d)Commission Directive 2001/59/EC (OJ No L225, 21.8.2001, p. 1) 28th adaptation to technical progress of the substances Directive;
(e)Commission Directive 2004/73/EC (OJ No L152, 30.4.04, p.1) 29th adaptation to technical progress of the substances Directive;
(f)Commission Directive 2001/60/EC (OJ No. L226, 22.8.2001, p. 5) 1st adaptation to technical progress of the preparations Directive; and
(g)Commission Directive 2006/8/EC (OJ No L19, 24.1.2006 p. 12) amending, for the purposes of their adaptation to technical progress, Annexes II, III and V to the preparations Directive.
4. The main purpose of the CLP Regulation is to adopt within the European Community the Globally Harmonised System of Classification and Labelling of Chemicals (GHS) published by the UN Social and Economic Council (Second Revised Edition ISBN-13:978-92-1-116957-7). The UN GHS is the result of an international agreement made at the United Nations Conference on Environment and Development in Rio de Janeiro in 1992, and the World Summit on Sustainable Development in Johannesburg in 2002. It sets out internationally accepted definitions and criteria to identify the hazards of chemicals and to communicate those hazards via labels and safety data sheets. The GHS is a voluntary international agreement and countries may keep national requirements that are not covered by the GHS provided that they do not contradict it.
5. The CLP Regulation progressively replaces, with transitional arrangements, the current Community classification and labelling system for hazardous chemicals with a new system based on the GHS. It replaces certain provisions of the substances Directive and the preparations Directive relating to the classification, packaging and labelling of substances and preparations through a two-stage process, first for substances and then for mixtures (currently referred to as “preparations”). Whilst many of the classification criteria, hazard symbols and labelling phrases are similar to the existing system, there are also some differences. The CLP Regulation requires dutyholders to classify, label and package hazardous chemicals before placing them on the market in accordance with its provisions.
6. Council Directive 2006/121/EC of Regulation (EC) No 1907/2006 (“REACH”) amends the substances Directive in order to adapt it to REACH. The major changes effected by Council Directive 2006/121/EC are to remove the provisions on the notification of new substances and on the provision of safety data sheets from the substances Directive. These provisions are now included in REACH and the changes are implemented by the REACH Enforcement Regulations 2008 (S.I. 2008/2852). The remaining changes effected by Council Directive 2006/121/EC are to delete references to Annex V of the substances Directive and replace references to it with references to the relevant parts of Commission Regulation (EC) No 440/2008 (OJ L142, 31.5.08, p. 1) laying down test methods pursuant to REACH and to amend certain other references. These remaining changes are implemented in these Regulations by the Approved Classification and Labelling Guide.
7. The terms and expressions used in the Regulations are defined in regulation 2 and the scope of the Regulations is described in regulation 3.
8. Regulations 4 to 10 implement the substances Directive and the preparations Directive. Regulation 11 implements the preparations Directive.
9. Regulation 4 describes the procedures for classifying dangerous substances and dangerous preparations. Regulation 5 refers to the safety data sheet provisions of REACH. Regulation 6 imposes requirements relating to the packaging of dangerous substances and dangerous preparations.
10. Regulation 7 imposes requirements in respect of the particulars that shall be shown on the labels for dangerous substances and dangerous preparations. Special labelling requirements are imposed in regulations 8 and 9. Regulation 10 imposes requirements in respect of the methods of marking and labelling of packages that contain dangerous substances or dangerous preparations.
11. Regulation 11 requires that the packaging of certain substances and preparations be provided with child resistant fastenings or tactile warning devices or both, and sets out the standards to which they shall conform.
12. Regulation 12 requires a person who classifies a dangerous preparation to retain the data used for the classification for at least three years after the preparation was last supplied.
13. Regulation 13 provides for transitional periods for compliance with the CLP Regulation. According to these arrangements, suppliers must classify both substances and mixtures according to regulation 4 until 1st June 2015, and must classify, label and package according to the CLP Regulation from 1st December 2010 for substances and 1st June 2015 for mixtures. However they may choose to classify, label and package in accordance with the CLP Regulation before 1st December 2010 for substances and 1st June 2015 for mixtures, in which case the requirements in regulations 6 to 11 on labelling and packaging cease to apply. Regulations 6 to 11 in any case cease to apply from 1st December 2010 for substances and 1st June 2015 for mixtures. Regulation 13 also describes the transitional arrangements for retention of data for dangerous preparations.
14. Regulation 14 makes provision for the enforcement of the Regulations and the CLP Regulation. Regulation 15 provides for a defence in specific circumstances in the case of contravention of the Regulations. Provision is made in regulation 16 to extend the application of the Regulations outside Great Britain. Revocations and amendments are set out in regulation 17 and 18.
15. Copies of the publications referred to in the Regulations are obtainable as follows—
(a)the approved classification and labelling guide (ISBN 0 7176 2369 6) from HSE Books (http://www.hsebooks.com or HSE Books, PO Box 1999, Sudbury, Suffolk CO10 2WA);
(b)The British and International Standards referred to in regulation 11 and Schedule 5 (relating to child resistant fastenings and tactile warning devices) from the British Standards Institution, 389 Chiswick High Road, London W4 4AL or online (http://www.bsi-global.com/upload/Standards%20&%20Publications/shop.html). 16. A copy of the regulatory impact assessment prepared in respect of these Regulations can be obtained from the Health and Safety Executive, Redgrave Court, Merton Road, Bootle, Merseyside L20 7HS. A copy of the transposition note in relation to the implementation of the Directives set out in paragraphs 2 and 3 can be obtained from the Health and Safety Executive, International Branch at the same address. Copies of these documents have been placed in the Library of each House of Parliament.
1972 c. 68. The power of the Minister to make regulations in relation to matters in or as regards Scotland is preserved by section 57(1) of the Scotland Act 1998 (c. 46).
1974 c. 37, as amended by S.I. 2008/960. Section 1(1)(c) was modified by the Health and Safety at Work etc. Act (Application to Environmentally Dangerous Substances) Regulations 2002, S.I. 2002/282. There are other amending instruments but none is relevant.
Section 11(3) is substituted by S.I. 2008/960.
OJ No L353, 31.12.08, p. 1.
OJ No L196, 16.8.67, p. 1.
OJ No L 200, 30.7.99, p. 1.
OJ No L 396, 30.12.06, p. 1.
OJ No L 204, 31.7.08, p. 1.
ISBN 0717623696.
1993 c. 51, to which there are amendments not relevant to these Regulations.
OJ No C146A, 15.6.90, p. 1.
OJ No C130, 10.5.93, p. 1.
S.I. 2005/1435, to which there is an amendment not relevant to these Regulations.
S.S.I. 2005/331, to which there are amendments not relevant to these Regulations.
1968 c. 67, as amended by S.I. 2006/2407. There are other amendments not relevant to these Regulations.
S.I. 2008/2297, to which there is an amendment not relevant to these Regulations.
S.I. 2004/1031, to which there are amendments not relevant to these Regulations.
S.I. 2008/1284, to which there are amendments not relevant to these Regulations.
S.I. 1996/972 as amended by S.S.I. 2004/112. There is a further amending instrument but this is not relevant to these Regulations.
S.I. 2005/1806 (W. 138), as amended by regulation 45 of S.I. 2007/3538.
S.I. 2005/894, to which there are amendments not relevant to these Regulations.
1970 c. 40, as amended by regulation 3(1)(a) of S.I. 2005/3281; regulation 3(1)(a) of S.S.I. 2005/605 and regulation 3(1)(a) of S.I. 2006/116. There are other amending instruments, but none is relevant.
S.I. 2002/618, to which there are amendments not relevant to these regulations.
S.I. 1997/2367, as amended by S.I. 2004/2110.
S.I. 2002/2786, as amended by S.I. 2008/2429.
1971/729, to which there are amendments not relevant to these Regulations.
Current edition (2009): ISBN 978-92-1-139131-2.
Current edition (2009): ISBN 978-92-1-139134-3.
Current edition (2009): ISBN 9789292311766.
Current edition (2008): ISBN: 978-92-801-4241-9.
Current edition: (2009): ISBN: 97888086206394.
S.I. 1986/1510, to which there are amendments not relevant to these Regulations.
S.I. 1998/494, to which there are amendments not relevant to these Regulations.
1987 c. 43 to which there amendments not relevant to these Regulations.
OJ No. L147, 9.6.1975, p. 40. Article 8 is amended to add paragraph 1a by Commission Directive 2008/47/EC, OJ No. L96, 9.4.2008, p. 15.
S.I. 1996/825, to which there are amendments not relevant to these Regulations.
S.I. 1998/494, to which there are amendments not relevant to these Regulations.
S.I. 2001/880, as amended by S.I. 2007/293. There are other amendments not relevant to these Regulations.
Options/Help
Print Options
PrintThe Whole Instrument
Mae deddfwriaeth ar gael mewn fersiynau gwahanol:
Y Diweddaraf sydd Ar Gael (diwygiedig):Y fersiwn ddiweddaraf sydd ar gael o’r ddeddfwriaeth yn cynnwys newidiadau a wnaed gan ddeddfwriaeth ddilynol ac wedi eu gweithredu gan ein tîm golygyddol. Gellir gweld y newidiadau nad ydym wedi eu gweithredu i’r testun eto yn yr ardal ‘Newidiadau i Ddeddfwriaeth’.
Gwreiddiol (Fel y’i Deddfwyd neu y’i Gwnaed): Mae'r wreiddiol fersiwn y ddeddfwriaeth fel ag yr oedd pan gafodd ei deddfu neu eu gwneud. Ni wnaed unrhyw newidiadau i’r testun.
Memorandwm Esboniadol
Mae Memoranda Esboniadol yn nodi datganiad byr o ddiben Offeryn Statudol ac yn rhoi gwybodaeth am ei amcan polisi a goblygiadau polisi. Maent yn ceisio gwneud yr Offeryn Statudol yn hygyrch i ddarllenwyr nad oes ganddynt gymhwyster cyfreithiol, ac maent yn cyd-fynd ag unrhyw Offeryn Statudol neu Offeryn Statudol Drafft a gyflwynwyd ger bron y Senedd o Fehefin 2004 ymlaen.
Rhagor o Adnoddau
Gallwch wneud defnydd o ddogfennau atodol hanfodol a gwybodaeth ar gyfer yr eitem ddeddfwriaeth o’r tab hwn. Yn ddibynnol ar yr eitem ddeddfwriaeth sydd i’w gweld, gallai hyn gynnwys:
- y PDF print gwreiddiol y fel deddfwyd fersiwn a ddefnyddiwyd am y copi print
- rhestr o newidiadau a wnaed gan a/neu yn effeithio ar yr eitem hon o ddeddfwriaeth
- manylion rhoi grym a newid cyffredinol
- pob fformat o’r holl ddogfennau cysylltiedig
- slipiau cywiro
- dolenni i ddeddfwriaeth gysylltiedig ac adnoddau gwybodaeth eraill
Asesiadau Effaith
Impact Assessments generally accompany all UK Government interventions of a regulatory nature that affect the private sector, civil society organisations and public services. They apply regardless of whether the regulation originates from a domestic or international source and can accompany primary (Acts etc) and secondary legislation (SIs). An Impact Assessment allows those with an interest in the policy area to understand:
- Why the government is proposing to intervene;
- The main options the government is considering, and which one is preferred;
- How and to what extent new policies may impact on them; and,
- The estimated costs and benefits of proposed measures.
Rhagor o Adnoddau
Defnyddiwch y ddewislen hon i agor dogfennau hanfodol sy’n cyd-fynd â’r ddeddfwriaeth a gwybodaeth am yr eitem hon o ddeddfwriaeth. Gan ddibynnu ar yr eitem o ddeddfwriaeth sy’n cael ei gweld gall hyn gynnwys:
- y PDF print gwreiddiol y fel gwnaed fersiwn a ddefnyddiwyd am y copi print
- slipiau cywiro
liciwch ‘Gweld Mwy’ neu ddewis ‘Rhagor o Adnoddau’ am wybodaeth ychwanegol gan gynnwys
- rhestr o newidiadau a wnaed gan a/neu yn effeithio ar yr eitem hon o ddeddfwriaeth
- manylion rhoi grym a newid cyffredinol
- pob fformat o’r holl ddogfennau cysylltiedig
- dolenni i ddeddfwriaeth gysylltiedig ac adnoddau gwybodaeth eraill