- Latest available (Revised)
- Original (As made)
The Preservatives in Food (Scotland) Regulations 1989
You are here:
- UK Statutory Instruments
- 1989 No. 581 (S. 66)
- Schedules only
More Resources
Status:
This is the original version (as it was originally made). This item of legislation is currently only available in its original format.
Regulation 2(1) and (2)
SCHEDULE 1
PART IPERMITTED PRESERVATIVES
| Column 1 | Column 2 | Column 3 | Column 4 |
|---|---|---|---|
| Permitted preservative specified in Schedule 2 | Serial Number | Alternative form in which the permitted preservative may be used (to be calculated as the permitted preservative shown in column 1) | Serial Number |
| Sorbic acid | E200 | Sodium sorbate | E201 |
| Potassium sorbate | E202 | ||
| Calcium sorbate | E203 | ||
| Benzoic acid | E210 | Sodium benzoate | E211 |
| Potassium benzoate | E212 | ||
| Calcium benzoate | E213 | ||
| Ethyl 4-hydroxybenzoate | E214 | Ethyl 4-hydroxybenzoate, sodium salt | E215 |
| Propyl 4-hydroxybenzoate | E216 | Propyl 4-hydroxybenzoate, sodium salt | E217 |
| Methyl 4-hydroxybenzoate | E218 | Methyl 4-hydroxybenzoate, sodium salt | E219 |
| Sulphur dioxide | E220 | Sodium sulphite | E221 |
| Sodium hydrogen sulphite | E222 | ||
| Sodium metabisulphite | E223 | ||
| Potassium metabisulphite | E224 | ||
| Calcium sulphite | E226 | ||
| Calcium hydrogen sulphite | E227 | ||
| Potassium bisulphite | E228 | ||
| Biphenyl | E230 | ||
| 2-Hydroxybiphenyl | E231 | Sodium biphenyl-2-yl oxide | E232 |
| 2-(Thiazol-4-yl) benzimidazole | E233 | ||
| Hexamine | E239 | ||
| Sodium nitrite | E250 | Potassium nitrite | E249 |
| Sodium nitrate | E251 | Potassium nitrate | E252 |
| Propionic acid | E280 | Sodium propionate | E281 |
| Calcium propionate | E282 | ||
| Potassium propionate | E283 | ||
| Nisin | 234 |
PART IISPECIFIC PURITY CRITERIA APPLICABLE TO PERMITTED PRESERVATIVES
| In the case of— | |
| E200 | Sorbic acid |
| E201 | Sodium sorbate |
| E202 | Potassium sorbate |
| E203 | Calcium sorbate |
| E210 | Benzoic acid |
| E211 | Sodium benzoate |
| E212 | Potassium benzoate |
| E213 | Calcium benzoate |
| E214 | Ethyl 4-hydroxybenzoate |
| Synonyms: Ethyl para-hydroxybenzoate | |
| Ethyl ester of p-hydroxybenzoic acid | |
| E215 | Ethyl 4-hydroxybenzoate, sodium salt |
| Synonyms: Sodium ethyl para-hydroxybenzoate | |
| Sodium ethyl p-hydroxybenzoate | |
| E216 | Propyl 4-hydroxybenzoate |
| Synonyms: Propyl para-hydroxybenzoate | |
| n-propyl p-hydroxybenzoate | |
| E217 | Propyl 4-hydroxybenzoate, sodium salt |
| Synonyms: Sodium propyl para-hydroxybenzoate | |
| Sodium n-propyl p-hydroxybenzoate | |
| E220 | Sulphur dioxide |
| E221 | Sodium sulphite (anhydrous or heptahydrate) |
| E222 | Sodium hydrogen sulphite |
| Synonyms: Acid sodium sulphite | |
| E223 | Sodium metabisulphite |
| E250 | Sodium nitrite |
| E251 | Sodium nitrate |
| E252 | Potassium nitrate |
| E280 | Propionic acid |
| E281 | Sodium propionate |
| E282 | Calcium propionate |
| the appropriate specific purity criteria contained in Directive 65/66/EEC of the Council(1). | |
| In the case of— | |
| E218 | Methyl 4-hydroxybenzoate |
| Synonyms: Methyl para-hydroxybenzoate | |
| Methyl p-hydroxybenzoate | |
| E219 | Methyl 4-hydroxybenzoate, sodium salt |
| Synonyms: Sodium methyl para-hydroxybenzoate | |
| Sodium derivative of methyl p-hydroxybenzoate | |
| E226 | Calcium sulphite |
| E227 | Calcium hydrogen sulphite |
| Synonym: Calcium bisulphite | |
| E233 | 2-(Thiazol-4-yl) benzimidazole |
| Synonyms: Thiabendazole | |
| 2-(4-thiazolyl) benzimidazole (thiabendazole) | |
| E239 | Hexamine |
| Synonym: Hexamethylenetetramine | |
| E249 | Potassium nitrite |
| E283 | Potassium propionate |
| the appropriate specific purity criteria contained in Directive 65/66/EEC of the Council(2). | |
| In the case of— | |
| E224 | Potassium metabisulphite |
| E230 | Biphenyl |
| E231 | 2-Hydroxybiphenyl |
| Synonym: Orthophenylphenol | |
| E232 | Sodium biphenyl-2-yl oxide |
| Synonym: Sodium orthophenylphenate | |
| the appropriate specific purity criteria contained in Directive 65/66/EEC of the Council(3). | |
| In the case of— | |
| E228 | Potassium bisulphite |
| Synonym: Potassium acid sulphite | |
| the appropriate specific purity criteria contained in Directive 65/66/EEC of the Council(4). | |
| In the case of— | |
| Nisin | |
| the criteria in the monograph for nisin contained in the Nutrition Meetings Report Series No. 45A (1969) of the United Nations' Food and Agriculture Organisation at page 53. |
PART IIIGENERAL PURITY CRITERIA APPLICABLE TO PERMITTED PRESERVATIVES EXCEPT WHERE OTHERWISE PROVIDED BY SPECIFIC PURITY CRITERIA
Each preservative shall not contain—
(a)more than 3 milligrams per kilogram of arsenic;
(b)more than 10 milligrams per kilogram of lead;
(c)more than 50 milligrams per kilogram of copper, or 25 milligrams per kilogram of zinc, or 50 milligrams per kilogram of any combination of copper and zinc.
Regulations 2(1) and 4(2)
SCHEDULE 2ARTICLES OF FOOD WHICH MAY CONTAIN PERMITTED PRESERVATIVE AND THE NATURE AND PROPORTION OF PERMITTED PRESERVATIVE IN EACH CASE
| Column 1 | Column 2 | Column 3 |
|---|---|---|
| Specified food | Permitted preservative | Except where otherwise stated milligrams per kilogram not exceeding— |
| Beer | Sulphur dioxide and either | 70 |
| benzoic acid or | 70 | |
| methyl 4-hydroxybenzoate or | 70 | |
| ethyl 4-hydroxybenzoate or | 70 | |
| propyl 4-hydroxybenzoate | 70 | |
| Beetroot, cooked and prepacked | Benzoic acid or | 250 |
| methyl 4-hydroxybenzoate or | 250 | |
| ethyl 4-hydroxybenzoate or | 250 | |
| propyl 4-hydroxybenzoate | 250 | |
| Bread | Propionic acid | As prescribed by the Bread and flour (Scotland) Regjulations 1984 |
| Cauliflower, canned | Sulphur dioxide | 100 |
| Cheese | Sorbic acid | 1,000 |
| Cheese, other than Cheddar, Cheshire, Grana-padano or Provolone type of cheeses or soft cheese | Sodium nitrate and sodium nitrite | 50 of which not more than 5 may be sodium nitrite, expressed in both cases as sodium nitrite |
| Provolone cheese | Hexamine | 25 (expressed as formaldehyde) |
| Chicory and coffee essence | Benzoic acid or | 450 |
| methyl 4-hydroxybenzoate or | 450 | |
| ethyl 4-hydroxybenzoate or | 450 | |
| propyl 4-hydroxybenzoate | 450 | |
| Christmas pudding | Propionic acid | 1,000 |
| Cider | Sulphur dioxide or | 200 |
| sorbic acid | 200 | |
| Coconut, desiccated | Sulphur dioxide | 50 |
| Colouring matter, except E150 | Benzoic acid or | 2,000 |
| Caramel, if in the form of a solution of a permitted colouring matter | methyl 4-hydroxybenzoate or | 2,000 |
| ethyl 4-hydroxybenzoate or | 2,000 | |
| propyl 4-hydroxybenzoate or | 2,000 | |
| sorbic acid | 1,000 | |
| The permitted colouring matter, E150 Caramel | Sulphur dioxide | 1,000 |
| Crabmeat, canned | Sulphur dioxide | 30 |
| Desserts, fruit based milk and cream | Sulphur dioxide or | 100 |
| sorbic acid | 300 | |
| Dessert sauces, fruit based with a total soluble solids countent of less than 75% | Sulphur dioxide or | 100 |
| benzoic acid or | 250 | |
| methyl 4-hydroxybenzoate or | 250 | |
| ethyl 4-hydroxybenzoate or | 250 | |
| propyl 4-hydroxybenzoate or | 250 | |
| sorbic acid | 1,000 | |
| The permitted miscellaneous additive, Dimethylphlysiloxane | Sulphur dioxide or | 1,000 |
| banzoic acid or | 2,000 | |
| methyl 4-hydroxybenzoate or | 2,000 | |
| ethyl 4-hydroxybenzoate or | 2,000 | |
| propyl 4-hydroxybenzoate or | 2,000 | |
| sorbic acid | 1,000 | |
| Enzymes: | Sulphur dioxide | 30,000 |
| Papain solid | Sulphur dioxide or | 5,000 |
| Papain, aqueous solutions | sorbic acid | 1,000 |
| Aqueous solutions of enzyme preparations not otherwise specified, including immobilised enzyme preparations in aqueous media | Sulphur dioxide or | 500 |
| benzoic acid or | 3,000 | |
| methyl 4-hydroxybenzoate or | 3,000 | |
| ethyl 4-hydroxybenzoate or | 3,000 | |
| propyl 4-hydroxybenzoate or | 3,000 | |
| sorbic acid | 3,000 | |
| Fat spreads consisting of an emulsion principally of water in oil with a fat content not exceeding 70% | Sorbic acid | 2,000 |
| Figs, dried | Sulphur dioxide or | 2,000 |
| sorbic acid | 500 | |
| Fillings and toppings for flour confectionary which consist principally of a sweetened oil and water emulsion with a minimum sugar solids content of 50% | Sorbic acid | 1,000 |
| Finings when sold by retail: | ||
| Wine finings | Sulphur dioxide | 12,500 |
| Beer finings | Sulphur dioxide | 50,000 |
| Flavourings | Sulphur dioxide or | 350 |
| benzoic acid or | 800 | |
| methyl 4-hydroxybenzoate or | 800 | |
| ethyl 4-hydroxybenzoate or | 800 | |
| propyl 4-hydroxybenzoate | 800 | |
| Flavouring syrups | Sulphur dioxide or | 350 |
| benzoic acid or | 800 | |
| methyl 4-hydroxybenzoate or | 800 | |
| ethyl 4-hydroxybenzoate or | 800 | |
| propyl 4-hydroxybenzoate | 800 | |
| Flour confectionery | Propionic acid or | 1,000 |
| sorbic acid | 1,000 | |
| Foam heading, liquid | Sulphur dioxide or | 5,000 |
| benzoic acid or | 10,000 | |
| methyl 4-hydroxybenzoate or | 10,000 | |
| ethyl 4-hydroxybenzoaate or | 10,000 | |
| propyl 4-hydroxybenzoate | 10,000 | |
| Freeze drinks | Sulphur dioxide or | 70 |
| benzoic acid or | 160 | |
| methyl 4-hydroxybenzoate or | 160 | |
| ethyl 4-hydroxybenzoate or | 160 | |
| propyl 4-hydroxybenzoate or | 160 | |
| sorbic acid | 300 | |
| Fruit based pie fillings | Sulphur dioxide or | 350 |
| benzoic acid or | 800 | |
| methyl 4-hydroxybenzoate or | 800 | |
| ethyl 4-hydroxybenzoate or | 800 | |
| propyl 4-hydroxybenzoate or | 800 | |
| sorbic acid | 450 | |
| Fruit, dried, other than prunes, or figs | Sulphur dioxide | 2,000 |
| Fruit, fresh: | ||
| Bananas | 2-(Thiazol-4-yl) benzimidazole | 3 |
| Citrus fruit | Biphenyl or | 70 |
| 2-hydroxybiphenyl or | 12 | |
| 2-(Thiazol-4-yl) beazimidazole | 10 | |
| Grapes | Sulphur dioxide | 15 |
| Fruit, fruit pulp or fruit purée (including tomatoes, tomato pulp, tomato paste and tomato purée) which, in each case, is not fresh or canned | Sulphur dioxide ir | 350 |
| benzoic acid or | 800 | |
| methyl 4-hydroxybenzoate or | 800 | |
| ethyl 4-hydroxybenzoate or | 800 | |
| propyl 4-hydroxybenzoate | 800 | |
| Fruit juices: | ||
| Any fruit juice or concentrated fruit juice mentioned in regulation 11(2) of the Fruit Juices and fruit Nectars (Scotland) Regulations 1977 | Sulphur dioxide | As prescribed by the Fruit Juiices and Fruit Nectars (Scotland) Regulations 1977 |
| Any other fruit juice or concentrated fruit juice | Sulphur dioxide or | 350 |
| benzoic acid or | 800 | |
| methyl 4-hydroxybenzoate or | 800 | |
| ethyl 4-hydroxybenzoate or | 800 | |
| propyl 4-hydroxybenzoate | 800 | |
| Fruit or plants (including flowers and seeds), crystallised, glacé drained (syruped) or candied peel or cut and drained (syruped) peel | Sulphur dioxide and either | 100 |
| benzoic acid or | 1,000 | |
| methyl 4-hydroxybenzoate or | 1,000 | |
| ethyl 4-hydroxybenzoate or | 1,000 | |
| propyl 4-hydroxybenzoate or | 1,000 | |
| sorbic acid | 1,000 | |
| Fruit pieces in stabilised syrup for use as ingredients of ice-cream or other edible ices | Sorbic acid | 1,000 |
| Fruit spread | Sulphur dioxide and | 100 |
| sorbic acid | 1,000 | |
| Garlic, powdered | Sulphur dioxide | 2,000 |
| Gelatin | Sulphur dioxide | 1,000 |
| Gelatin capsules | Sorbic acid | 3,000 |
| Ginger, dry root | Sulphur dioxide | 150 |
| Glucose drinks containing not less than 235 grammes of glucose syrup per litre of the drink | Sulphur dioxide or | 350 |
| benzoic acid or | 800 | |
| methyl 4-hydroxybenzoate or | 800 | |
| ethyl 4-hydroxybenzoate or | 800 | |
| propyl 4-hydroxybenzoate | 800 | |
| Grape juice products (unfermented, intended for sacramental use) | Sulphur dioxide and either | 70 |
| benzoic acid or | 2,000 | |
| methyl 4-hydroxybenzoate | 2,000 | |
| ethyl 4-hydroxybenzoate or | 2,000 | |
| propyl 4-hydroxybenzoate | 2,000 | |
| Grape juice, concentrated, intended for home wine making and labelled as such | Sulphur dioxide | 2,000 |
| Hamburgers or similar products | Sulphur dioxide | 450 |
| Herring, marinated | ||
| —whose pH does not exceed 4.5 | Benzoic acid or | 1,000 |
| methyl 4-hydroxybenzoate or | 1,000 | |
| ethyl 4-hydroxybenzoate or | 1,000 | |
| propyl 4-hydroxybenzoate | 1,000 | |
| —whose pH exceeds 4.5 | Hexamine and either | 50 |
| benzoic acid or | 1,000 | |
| methyl 4-hydroxybenzoate or | 1,000 | |
| ethyl 4-hydroxybenzoate or | 1,000 | |
| propyl 4-hydroxybenzoate | 1,000 | |
| Hops, dried, sold by retail | Sulphur dioxide | 2,000 |
| Horseradish, fresh grated, and horseradish sauce | Sulphur dioxide or | 200 |
| benzoic acid or | 250 | |
| methyl 4-hydroxybenzoate or | 250 | |
| ethyl 4-hydroxybenzoate or | 250 | |
| propyl 4-hydroxybenzoate | 250 | |
| Jam and other products described in column 2 of Schedule 1 to the Jam and Similar Products (Scotland) Regulations 1981: | ||
| Reduced sugar jam, reduced sugar jelly and reduced sugar marmalade | Sulphur dioxide and benzoic acid or methyl 4-hydroxybenzoate or ethyl 4-hydroxybenzoate or propyl 4-hydroxybenzoate or sorbic acid | } As prescribed in the Jam and similar Products (Scotland) Regjulations 1981 |
| Any other product described in column 2 of Schedule 1 to the Jam and Similar Products (Scotland) Regulations 1981 | Sulphur dioxide | As prescribed in the Jam and Similar Products (Scotland) Regulations 1981 |
| Mackerel, marinated. | ||
| —whose pH does not exceed 4.5 | Benzoic acid or | 1,000 |
| methyl 4-hydroxybenzoate or | 1,000 | |
| ethyl 4-hydroxybenzoate or | 1,000 | |
| propyl 4-hydroxybenzoate | 1,000 | |
| —whose pH exceeds 4.5 | Hexamine and either | 50 |
| benzoic acid or | 1,000 | |
| methyl 4-hydroxybenzoate or | 1,000 | |
| ethyl 4-hydroxybenzoate or | 1,000 | |
| propyl 4-hydroxybenzoate | 1,000 | |
| Mallow, chocolate covered | Sorbic acid | 1,000 (calculated on the weight of the mallow and chocolate together) |
| Meat, cured (including cured meat products): | ||
| Cured meat (including cured meat products) packed in a sterile pack, whether or not it has been removed from the pack | Sodium nitrate and sodium nitrite | } 150, of which not more than 50 may be sodium nitrite, expressed in both cases as sodium nitrite |
| Acidified and/or fermented cured meat products (including Salami and similar products) not packed in a sterile pack | Sodium nitrate and sodium nitrite | } 400, of which not more than 50 may be sodium nitrite, expressed in both cases as sodium nitrite |
| Uncooked bacon and ham: cooked bacon and ham that is not, and has not been, packed in any hermetically sealed container | Sodium nitrate and sodium nitrite | } 500, of which not more than 200 may be sodium nitrite, expressed in both cases as sodium nitrite |
| Any cured meat or cured meat product not specified above | Sodium nitrate and sodium nitrite | } 250, of which not more than 150 may be sodium nitrite, expressed in both cases as sodium nitrite |
| Mushrooms, frozen | Sulphur dioxide | 50 |
| Nut pastes, sweetened | Sorbic acid | 1,000 |
| Olives, pickled | Sulphur dioxide or | 100 |
| benzoic acid or | 250 | |
| methyl 4-hydroxybenzoate or | 250 | |
| ethyl 4-hydroxybenzoate or | 250 | |
| propyl 4-hydroxybenzoate or | 250 | |
| sorbic acid | 500 | |
| Peas, garden, canned, containing no added colouring matter | Sulphur dioxide | 100 |
| Pectin,m liquid | Sulphur dioxide | 250 |
| Perry | Sulphur dioxide or | 200 |
| sorbic acid | 200 | |
| Pickles, other than pickled olives | Sulphur dioxide or | 100 |
| benzoic acid or | 250 | |
| methyl 4-hydroxybenzoate or | 250 | |
| ethyl 4-hydroxybenzoate or | 250 | |
| propyl 4-hydroxybenzoate or | 250 | |
| sorbic acid | 1,000 | |
| Potatoes, raw, peeled | Sulphur dioxide | 50 |
| Prawns, shrimps and scampi, other than prawns and shrimps in brine | Sulphur dioxide | 200 in the edible part |
| Prawns and shrimps in brine | Sulphur dioxide and either | 200 in the edible part |
| sorbic acid or benzoic acid and either | 2,000 | |
| ethyl 4-hydroxybenzoate or | 300 | |
| propyl 4-hydroxybenzoate or | 300 | |
| methyl 4-hydroxybenzoate | 300 | |
| Preparations of saccharin, sodium saccharin or calcium sccharin and water only | Benzoic acid and either | 750 |
| methyl 4-hydroxybenzoate or | 250 | |
| ethyl 4-hydroxybenzoate or | 250 | |
| propyl 4-hydroxybenxoate | 250 | |
| Prunes | Sulphur dioxide or | 2,000 |
| sorbic acid | 1,000 | |
| Salad cream (including mayonnaise) and salad dressing | Sulphur dioxide or | 100 |
| benzoic acid or | 250 | |
| methyl 4-hydroxybenzoate or | 250 | |
| ethyl 4-hydroxybenzoate or | 250 | |
| propyl 4-hydroxybenzoate or | 250 | |
| sorbic acid | 1,000 | |
| Sambal oelek | Benzoic acid and | 850 |
| sorbic acid | 1,000 | |
| Sauces, other than horseradish sauce | Sulphur dioxide or | 100 |
| benzoic acid or | 250 | |
| methyl 4-hydroxybenzoate or | 250 | |
| ethyl 4-hydroxybenzoate or | 250 | |
| propyl 4-hydroxybenzoate or | 250 | |
| sorbic acid | 1,000 | |
| Sausages or sausage meat | Sulphur dioxide | 450 |
| Snack meals, concentrated with a moisture content of not less than 15% and not more than 60% | Sorbic acid and | 1,500 |
| methyl 4-huydroxybenzoate | 175 | |
| Soft drinks for consumption after dilution not otherwise specified in this Schedule | Sulphur dioxide or | 350 |
| benzoic acid or | 800 | |
| methyl 4-hydroxybenzoate or | 800 | |
| ethyl 4-hydroxybenzoate or | 800 | |
| propyl 4-hydroxybenzoate or | 800 | |
| sorbic acid | 1,500 | |
| Soft drinks for consumption without dilution not otherwise specified in this Schedule | Sulphur dioxide or | 70 |
| benzoic acid or | 160 | |
| methyl 4-hydroxybenzoate or | 160 | |
| ethyl 4-hydroxybenzoate or | 160 | |
| propyl 4-hydroxybenzoate or | 160 | |
| sorbic acid | 300 | |
| Soup concentrates with a moisture content of not less than 25% and not more than 60% | Sorbic acid and | 1,500 |
| methyl 4-hydroxybenzoate | 175 | |
| Starches, including modified starches | Sulphur dioxide | 100 |
| Sugars: | ||
| Specified sugar products | Sulphur dioxide | As prescribed by the Specified Sugar Products (Scotland) Regulations 1976 |
| Hydrollysed starches (other than specified sugar products) | Sulphur dioxide | 400 |
| Other sugars except lactose | Sulphur dioxide | 70 |
| Tea extract, liquid | Benzoic acid or | 450 |
| Methyl 4-hydroxybenzoate or | 450 | |
| ethyl 4-hydroxybenzoate or | 450 | |
| propyl 4-hysroxybenzoate | 450 | |
| Vegetables, dehydrated: | ||
| Brussels sprouts | Sulphur dioxide | 2,500 |
| Cabbage | Sulphur dioxide | 2,500 |
| Potato | Sulphur dioxide | 550 |
| Others | Sulphur dioxide | 2,000 |
| Vinegar: | ||
| Cider or wine vinegar | Sulphur dioxide | 200 |
| Other | Sulphur dioxide | 70 |
| Wine (including alcoholic cordials) other than Community controlled wine | Sulphur dioxide and | 450 milligrams per litre |
| sorbic acid | 200 milligrams per litre | |
| Yoghurt, fruit | Sulphur dioxide or | 60 |
| benzoic acid or | 120 | |
| methyl 4-hydroxybenxzoate or | 120 | |
| ethyl 4-hydroxybenzoate or | 120 | |
| propyl 4-hydroxybenzoate or | 120 | |
| sorbic acid | 300 |
Regulations 4(4)and 6(2)
SCHEDULE 3LABELLING OF PERMITTED PRESERVATIVES
1.—(1) Each container to which regulation 6(2) applies shall bear a label on which is printed a true statement—
(a)in respect of each permitted preservative present, of the serial number, if any, as specified in relation thereto in column 2 or 4 of Part I of Schedule 1, and of the common or usual name or an appropriate designation of that permitted preservative;
(b)where any other substance or substances is or are present, of the common or usual name or an appropriate designation of each such substance; and
(c)where two or more of the substances referred to in paragraphs 1(1)(a) and (b) of this Schedule are present, of the proportion of each such substance present, save that the label shall only have printed on it a statement of the proportion of any substance present, other than a permitted preservative, if any regulations, other than these Regulations or any amendment to these Regulations, made under the Act contain a requirement to that effect.
(2) The said statement shall be headed or preceded by the words “for foodstuffs (restricted use)”.
2. Any statement required by the preceding paragraph—
(a)shall be clear and legible;
(b)shall be in a conspicuous position on the label which shall be marked on, or securely attached to, the container in such a manner that it will be readily discernible and easily read by an intending purchaser under normal conditions of purchase;
(c)shall not be in any way hidden or obscured or reduced in conspicuousness by any other matter, whether pictorial or not, appearing on the label.
3. The figures and the letters in any statement to which the preceding paragraph applies—
(a)shall be in characters of uniform colour and size (being not less than 1.5 millimetres in height for a label on a container of which the greatest dimension does not exceed 12 centimetres, and not less than 3 millimetres in height for a label on a container of which the greatest dimension exceeds 12 centimetres), but so that the initial letter of any word may be taller than any other letter in the word;
(b)shall appear on a contrasting ground, so however that where there is no ground other than such as is provided by a transparent container and the contents of that container are visible behind the letters, those contents shall be taken to be the ground for the purposes of this paragraph;
(c)shall be within a surrounding line and no other written or pictorial matter shall appear within that line.
4.—(1) There shall be printed on each document to which regulation 4(4) refers a true statement—
(a)of the common or usual name or an appropriate designation of the food to which the document relates;
(b)in respect of each permitted preservative present in the food to which the document relates, of the serial number, if any, as specified in relation thereto in column 2 or 4 of Part I of Schedule 1, and of the common or usual name or an appropriate designation of that permitted preservative; and
(c)of the proportion of each permitted preservative present in the food to which the document relates.
(2) The said statement shall include the words “Not for retail sale”.
5. Any statement required by the preceding paragraph shall be clear and legible and the figures and the letters in any such statement—
(a)shall be in characters of uniform colour and size and not less than 3 millimetres in height, but so that the initial letter of any word may be taller than any other letter in the word;
(b)shall appear on a contrasting ground;
(c)shall be within a surrounding line and no other written or pictorial matter shall appear within that line.
6. For the purposes of this Schedule—
(a)the height of any lower case letter shall be taken to be the x-height thereof, disregarding any ascender or descender thereof;
(b)any requirement that figures or letters shall be of uniform height, colour or size shall be construed as being subject to the saving that any inconsiderable variation in height, colour or size, as the case may be, may be disregarded.
Regulation 8(1)
SCHEDULE 4SAMPLING OF CITRUS FRUIT TREATED WITH BIPHENYL, 2-HYDROXYBIPHENYL OR SODIUM BIPHENYL-2-YL OXIDE
PART I
Procuring of sample
1. A sample shall be procured using scientific methods which ensure that the sample is representative of the lot to which it relates.
2. A sample shall satisfy at least the following requirements—
(a)in the case of goods packaged in crates, boxes or similar containers—
| Number of containers in the lot | Up to 1,000 | Above 1,000 |
|---|---|---|
| Minimum number of containers to be sampled ... ... ... ... | 3 | 4 |
| Mass, in kg., of fruit to be treated as sample per container ... ... ... ... | 2 | 2 |
(b)in the case of goods in bulk—
| Number of containers in the lot | Up to 1,000 | Above 1,000 |
|---|---|---|
| Mass of bath in kh ... ... ... ... | 500 | 500 |
| Mass, in kg, to be treated as sample | 6 | 8 |
3. In this Part of this Schedule, the expression “lot” means a part of a consignment, which part has throughout the same characteristics such as variety of fruit, degree of ripeness and type of packaging.
PART II
Packaging and delivery of sample
1. Each part of the sample shall be placed in an air-tight container which shall be sealed.
2. Each part of the sample to be submitted for analysis shall be delivered so packaged as quickly as possible to the test laboratory.
Regulation 8(2)
SCHEDULE 5ANALYSIS OF CITRUS FRUIT TREATED WITH BIPHENYL, 2-HYDROXYBIPHENYL OR SODIUM BIPHENYL-2-YL OXIDE
PART IQualitative analysis for residues of biphenyl, 2-hydroxybiphenyl and sodium biphenyl-2-yl oxide in citrus fruit
Purpose and scope
1. The method described below enables the presence of residues of biphenyl, 2-hydroxybiphenyl (orthophenylphenol) or sodium biphenyl-2-yl oxide (sodium orthophenylphenate) in the peel of citrus fruit to be detected. The sensitivity limit of this method, in absolute terms, is approximately 5 μg for biphenyl and 1 μg for 2-hydroxybiphenyl or sodium biphenyl-2-yl oxide, which is the equivalent of 5 mg of biphenyl and 1 mg of 2-hydroxybiphenyl respectively in the peel of 1 kg of citrus fruit.
Principle
2. An extract is prepared from the peel using dichloromethane in an acid medium. The extract is concentrated and separated by thin layer chromatography using silica gel. The presence of biphenyl, 2-hydroxybiphenyl or sodium biphenyl-2-yl oxide is shown by fluorescence and colour tests.
Reagents
3. The following reagents shall be used—
(a)cyclohexane (analytical reagent grade);
(b)dichloromethane (analytical reagent grade);
(c)hydrochloric acid 25 per centum (weight/volume);
(d)silica gel GF 254 (Merck or equivalent);
(e)0.5 per centum (weight/volume) solution of 2, 4, 7-trinitrofluorenone (TNF) (Fluka, BDH or equivalent) in acetone;
(f)0.1 per centum (weight/volume) solution of 2, 6-dibromo-p-benzoquinone-chlorimine in ethanol (stable for up to one week if kept in the refrigerator);
(g)concentrated solution of ammonia, specific gravity: 0.9;
(h)standard 1 per centum (weight/volume) solution of pure biphenyl in cyclohexane;
(j)standard 1 per centum (weight/volume) solution of pure 2-hydroxybiphenyl in cyclohexane.
Apparatus
4. The following apparatus shall be used—
(a)a mixer;
(b)a 250 ml flask with ground glass joint and with a reflux condenser;
(c)a reduced pressure evaporator;
(d)micropipettes;
(e)a thin layer chromatographic apparatus with plates measuring 20x20 cm;
(f)an ultra-violet lamp (254 nm), the intensity of which should be such that a spot of 5 mg of biphenyl is visible;
(g)equipment for pulverising reagents;
(h)an oven.
Method of analysis
5. The analysis shall be carried out as follows—
(a)Preparation and extraction: All the fruit in the sample for analysis is cut in half. Half of each piece of fruit is kept for quantitative determination of the residue of any biphenyl or 2-hydroxybiphenyl present. Pieces of peel are taken from the other halves to give a sample of about 80 g. These pieces are chopped, crushed in the mixer and placed in the 250 ml flask; to this is added 1 ml of 25 per centum hydrochloric acid and 100 ml dichloromethane. The mixture is heated under reflux for 10 minutes. After cooling and rinsing of the condenser with about 5 ml of dichloromethane, the mixture is filtered through a fluted filter. The solution is transferred to the evaporator and some anti-bumping granules are added. The solution is concentrated at reduced pressure at a temperature of 60C to a final volume of about 10 ml. If a rotary evaporator is used, the flask should be kept in a fixed position to avoid loss of biphenyl through the formation of a film of the product on the upper wall of the flask.
(b)Chromatography: 30 g of silica gel and 60 ml of water are placed in a mixer and mixed for one minute. The mixture is then spread on to 5 chromatographic plates to form a layer approximately 0.25 mm thick. The plates covered with this layer are subjected to a stream of hot air for 15 minutes and then placed in an oven where they are kept for 30 minutes at a temperature of 110C.
After cooling, the surface layer of each plate is divided into lanes, 2 cm wide, by parallel lines penetrating the silica gel down to the surface of the glass plate. 50 μl of the extract to be analysed are applied to each lane as a narrow band of contiguous spots approximately 1.5 cm from the lower edge of the plate. At least one lane is kept for the controls consisting of a spot of 1 ul (that is, 10 μg) of the standard solutions of biphenyl and 2-hydroxybiphenyl, one standard per lane. The chromatographic plates are developed in a mixture of cyclohexane and dichloromethane (25:95) in tanks previously lined with filter paper.
(c)Detection and identification: The presence of biphenyl and 2-hydroxybiphenyl is shown by the appearance of spots in ultra-violet light (254 nm). The sodium biphenyl-2-yl oxide will have been converted to 2-hydroxybiphenyl during the extraction in an acid medium, and its presence cannot therefore be distinguished from that of 2-hydroxybiphenyl. The products are identified in the following manner—
(i)biphenyl gives a yellow spot in daylight when sprayed with the TNF solution;
(ii)2-hydroxybiphenyl gives a blue spot when sprayed with the solution of 2,6-dibromo-p-benzoquinonechlorimine, followed by rapid passage through a stream of hot air and exposure to an ammonia-saturated atmosphere.
PART IIQuantitative analysis of the residues of biphenyl in citrus fruit
Purpose and scope
1. The method described below gives a quantitative analysis of the residues of biphenyl in whole citrus fruit. The accuracy of the method is ± 10 per centum for a biphenyl content greater than 10 mg per kg of fruit.
Principle
2. After distillation in an acid medium and extraction by cyclohexane, the extract is subject to thin layer chromatography on silica gel. The chromatogram is developed and the biphenyl is eluted and determined spectrophotometrically at 248 nm.
Reagents
3. The following reagents shall be used—
(a)concentrated sulphuric acid solution;
(b)silicone-based anti-foaming emulsion;
(c)cyclohexane (analytical reagent grade);
(d)hexane (analytical reagent grade);
(e)ethanol (analytical reagent grade);
(f)anhydrous sodium sulphate;
(g)silica gel GF 254 (Merck or equivalent);
(h)standard 1 per centum (weight/volume) solution of pure biphenyl in cyclohexane: dilute with cyclohexane to obtain the following three solutions—
(i)0.6 μg/μl;
(ii)1 μg/μl;
(iii)1.4 μg/μl.
Apparatus
4. The following apparatus shall be used—
(a)a 1 litre mixer;
(b)a 2 litre distillation flask with a modified Clevenger-type separator as shown in the diagram in Schedule 6 and a cooled reflux condenser;
(c)a 10 ml graduated flask;
(d)50 /μl micropipettes;
(e)a thin layer chromatographic apparatus with 20x20 cm plates;
(f)an oven;
(g)a centrifuge with 15 ml conical tubes;
(h)an ultra-violet spectrophotometer.
Method of analysis
5. The analysis shall be carried out as follows—
(a)Preparation and extraction: All the fruit in the sample for analysis is cut in half. Half of each piece of fruit is kept for qualitative analysis for residues of biphenyl, 2-hydroxybiphenyl or sodium biphenyl-2-yl oxide. The other halves are put all together and shredded in a mill or crushed until a homogeneous mixture is obtained. From this at least two sub-samples of 200 g are taken for analysis in the following manner. Each sub-sample is placed in a mixer with 100 ml of water and mixed at slow speed for several seconds. Water is added until the volume of the mixture reaches 3/4 of the capacity of the mixer, and the mixture is then mixed for 5 minutes at full speed. The resulting puree is transferred to the 2 litre distillation flask. The mixer is rinsed with water and the rinsings added to the contents of the flask. (The total quantity of water to be used in mixing and rinsing is 1 litre.) To the mixture are added 2 ml sulphuric acid, 1 ml anti-foaming emulsion and several anti-bumping granules. The separator and reflux condenser are fitted on to the flask. Distilled water is poured into the separator until the water level is well past the lower arm of the lateral return tube, followed by 7 ml cyclohexane. Distillation is carried out for about 2 hours. The lower aqueous layer in the separator is discarded and the upper layer is collected in the 10 ml graduated flask. The separator is rinsed with about 1.5 ml of cyclohexane and the rinsings added to the contents of the flask, which are then brought up to volume with cyclohexane. Finally a little anhydrous sodium sulphate is added and the mixture is shaken.
(b)Chromatography: 30 g of silica gel and 60 ml of water are placed in a mixer and mixed for one minute. The mixture is then spread on to 5 chromatographic plates to form a layer approximately 0.25 mm thick. The plates covered with this layer are subjected to a stream of hot air for 15 minutes and then placed in an oven where they are kept for 30 minutes at a temperature of 110C. After cooling, the surface layer of each plate is divided into 4 lanes, 4.5 cm wide, by parallel lines penetrating the silica gel down to the surface of the glass plate. 50 μl of the extract to be analysed are applied to one lane of each plate as a narrow band of contiguous spots approximately 1.5 cm from the lower edge of the plate. 50 μl of the standard solutions (i), (ii) and (iii), corresponding respectively to 30, 50 and 70 μg levels of biphenyl are applied in the same way to the three remaining lanes, one solution to each lane.
If a large number of samples are being analysed at one time, standard solutions need not be applied to every plate. Reference may be made to a standard curve provided that this curve has been prepared from the average values obtained from 5 different plates to which the same standard solutions have been applied.
(c)Development of chromatograms and elution: The chromatograms are developed with hexane to a height of 17 cm in tanks previously lined with filter paper. The plates are air dried. By illuminating the plates with ultra-violet light (254 nm), the areas of silica gel containing biphenyl are located and marked off in rectangles of equal area.
The entire layer of silica gel within the areas thus marked off is immediately scraped from the plate with a spatula. The biphenyl is extracted by mixing the silica gel with 10 ml of ethanol and shaking several times over a period of 10 minutes. The mixture is transferred to the centrifuge tubes and centrifuged for 5 minutes at 2,500 revolutions per minute.
A control sample of silica gel is taken by the same method using an area of the same size. If a series of analyses are made, this control area is taken from an unused lane of a plate and below the solvent front; if a single analysis is made the control sample is taken from an area below one of the positions at which the standard biphenyl is located.
(d)Spectrophotometric determination: The supernatant liquid is decanted into the spectrophotometer cells and the absorption determined at 248 nm against a control extract from a chromatographic area free from biphenyl.
Calculation of results
6. A standard curve is drawn, plotting the biphenyl values of 30, 50 and 70 μg against the corresponding absorptions, as determined on the spectrophotometer. This gives a straight line which passes through the origin. This graph allows the biphenyl content of the samples to be read directly in mg per kg from the absorption value of their extracts.
PART IIIQuantitative analysis of the residues of 2-hydroxybiphenyl and sodium biphenyl-2-yl oxide in citrus fruit
Purpose and scope
1. The method described below enables a quantitative analysis of the residues of 2-hydroxybiphenyl and sodium biphenyl-2-yl oxide in whole citrus fruit to be made. The method gives results which for a 2-hydroxybiphenyl or sodium biphenyl-2-yl oxide content of the order of 12 mg per kg are low by an average value of between 10 per centum and 20 per centum.
Principle
2. After distillation in an acid medium and extraction by di-isopentyl ether, the extract is purified and treated with a solution of 4-aminophenazone. A red colour develops, the intensity of which is measured spectrophotometrically at 510 nm.
Reagents
3. The following reagents shall be used—
(a)70 per centum (weight/weight) orthophosphoric acid;
(b)silicone-based anti-foaming emulsion;
(c)di-isopentyl ether (analytical reagent grade);
(d)purified cyclohexane: shake 3 times with a 4 per cent (weight/volume) solution of sodium hydroxide, wash 3 times with distilled water;
(e)4 per centum (weight/volume) sodium hydroxide solution;
(f)buffer solution at pH 10.4: into a 2 litre graduated flask put 6.64 g of boric acid, 8.00 g of potassium chloride and 93.1 ml of N sodium hydroxide solution; mix and bring up to calibration mark with distilled water;
(g)reagent I: dissolve 1.0 g of 4-aminophenazone (4-amino-2, 3-dimethyl-1-phenyl-5-pyrazolone; 4-aminoantipyrin) in 100 ml of distilled water;
(h)reagent II: dissolve 2.0 g of potassium ferricyanide in 100 ml of distilled water. Reagents I and II must be kept in brown glass flasks and are only stable for approximately 14 days;
(j)silica gel;
(k)standard solution: dissolve 10 mg of pure 2-hydroxybiphenyl in 1 ml of 0.1 N NaOH; dilute to 100 ml with a 0.2 M sodium borate solution (1 ml = 100 ug 2-hydroxybiphenyl). For the standard curve, dilute 1 ml to 10 ml with the buffer solution.
Apparatus
4. The following apparatus shall be used—
(a)a shredding or crushing mill;
(b)a mixer;
(c)a 1 litre distillation flask with a modified Clevenger-type separator as shown in the diagram in Schedule 6 and a reflux condenser;
(d)an electrically controlled heating mantle;
(e)a 200 ml separating funnel;
(f)graduated cylinders of 25 and 100 ml;
(g)graduated flasks of 25 and 100 ml;
(h)1 to 10 ml pipettes;
(j)0.5 ml graduated pipettes;
(k)a spectrophotometer with 4 or 5 cm cells.
Method of Analysis
5. All the fruit in the sample for analysis is cut in half. Half of each piece of fruit is kept for qualitative analysis for residues of biphenyl, 2-hydroxybiphenyl or sodium biphenyl-2-yl oxide. The other halves are put all together and shredded in a mill or crushed until a homogeneous mixture is obtained. From this at least two sub-samples of 250 g are taken for analysis in the following manner.
Each sub-sample is placed in a mixer with 500 ml of water and mixed until a very fine homogeneous mixture is obtained in which the oily cells are no longer perceptible. A sample of 150 to 300 g of the puree is taken, depending on the presumed 2-hydroxybiphenyl content and placed in the 1 litre distillation flask with a quantity of water sufficient to dilute the mixture to 500 g in the flask. After the addition of 10 ml of 70 per centum orthophosphoric acid, several anti-bumping granules and 0.5 ml of anti-foaming emulsion, the separator and the reflux condenser are fitted on to the flask. 10 ml of di-isopentyl ether are placed in the separator and the flask is heated gently in the electrically controlled heating mantle until the mixture boils. Emulsion formation is minimised if the mixture is boiled gently for the first 10 to 20 minutes. The rate of heating is then gradually increased until the mixture boils steadily and one drop of water reaches the trapping solvent every 3 to 5 seconds. After distilling for 6 hours, the contents of the separator are poured into the 200 ml separating funnel, and the separator and the condenser are rinsed with 60 ml of cyclohexane and then with 60 ml of water. The rinsings are added to the contents of the separating funnel. The mixture is shaken vigorously and when the phases have separated the aqueous phase is discarded.
To extract the 2-hydroxybiphenyl, the organic phase is shaken vigorously 5 times, each time for 3 minutes, with 10 ml of 4 per centum sodium hydroxide. The alkaline solutions are combined, adjusted to pH 9-10 with orthophosphoric acid in the presence of phenolphthalein paper, and diluted to 100 ml with distilled water. A pinch of silica gel is added in order to clarify the solution which will have a slightly cloudy appearance. The solution is then shaken and filtered through a dry, fine-grain filter. Since colouring is developed with the maximum of accuracy and precision using quantities of 2-hydroxybiphenyl of between 10 and 70 ug an aliquot sample of between 0.5 and 10 ml of solution is taken with a pipette, taking into account the quantities of 2-hydroxybiphenyl which might be expected to be found. The sample is placed in a 25 ml graduated flask; to this are added 0.5 ml of reagent I, 10 ml of the buffer solution and then 0.5 ml of reagent II. The mixture is made up to the calibration mark with the buffer solution and shaken vigorously.
After 5 minutes the absorption of the red colouring at 510 nm. is measured spectrophotometrically against a control containing no extract. The colour does not lose intensity within 30 minutes. Evaluation is made by reference to a standard curve drawn from determinations using the standard 2-hydroxybiphenyl solution under the same conditions.
Observations
6. For each analysis it is recommended that the spectrophotometric determination be made with two different volumes of the neutralised alkaline extract.
Untreated citrus fruit gives by this method a “blank” reading of up to 0.5 mg per kg for oranges and 0. 8 mg per kg for lemons.
Regulation 8(3)
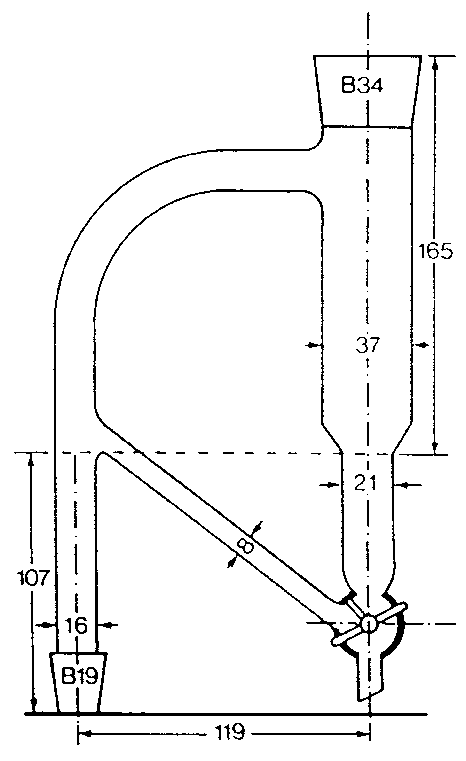
SCHEDULE 6DIAGRAM OF A MODIFIED CLEVENGER-TYPE SEPARATOR
Regulation 13
SCHEDULE 7
| Regulations revoked | References | Extent of revocation |
|---|---|---|
| The Preservatives in Food (Scotland) Regulations 1979 | S.I. 1979/1073 | All the Regulations |
| The Preservatives in Food (Scotland) Amendment Regulations 1980 | S.I. 1980/1232 | All the Regulations |
| The Jam and Similar Products (Scotland) Regulations 1981 | S.I. 1981/1320 | Regulation 20 and Schedule 5 |
| The Preservatives in Food (Scotland) Amendment Regulations 1982 | S.I. 1982/516 | All the Regulations |
| The Fruit Juices and Fruit Nectars (Scotland) Amendment Regulations 1982 | S.I. 1982/1619 | Regulation 9 |
| The Food and Drugs (Scotland) Act 1956 (Transfer of Enforcement Functions) Regulations 1983 | S.I. 1983/270 | The reference in Schedule 2 to the Preservatives in Food (Scotland) Regulation 1989 |
| The Sweeteners in Food (Scotland) Regulations 1983 | S.I. 1983/1497 | Schedule 2, paragraph 5 |
| The Bread and Flour (Scotland) Regulations 1984 | S.I. 1984/1518 | Schedule 6, paragraph 4 |
| The Food (Revisiion of Penalties and Mode of Trial) (Scotland) Regulation 1985 | S.I. 1985/1068 | The reference in Schedules 1 and 2 to the Preservatives in Food (Scotland) Regulations 1979 |
O.J. No. 22, 9.2.65, p.373/65 (O.J./S.E. 1965-1966, p.25); relevant amending Directives are 67/428/EEC of the Council-O.J. No. 148, 11.7.67, p.10 (O.J./S.E. 1967, p.178); 76/463/EEC of the Council -O.J. No. L126, 14.5.76, p.33.
The relevant amending Directive is 76/463/EEC of the Council -O.J. No. L126, 14.5.76, p.33.
The relevant amending Directive is 67/428/EEC of the Council -O.J. No. 148, 11.7.67, p.10 (O.J./S.E. 1967, p.178).
The relevant amending Directive is 86/604/EEC of the Council -O.J. No. L352, 13.12.1986, p.45.
Options/Help
Print Options
PrintThe Whole Instrument
PrintThe Schedules only
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As Enacted or Made): The original version of the legislation as it stood when it was enacted or made. No changes have been applied to the text.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as enacted version that was used for the print copy
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as made version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources